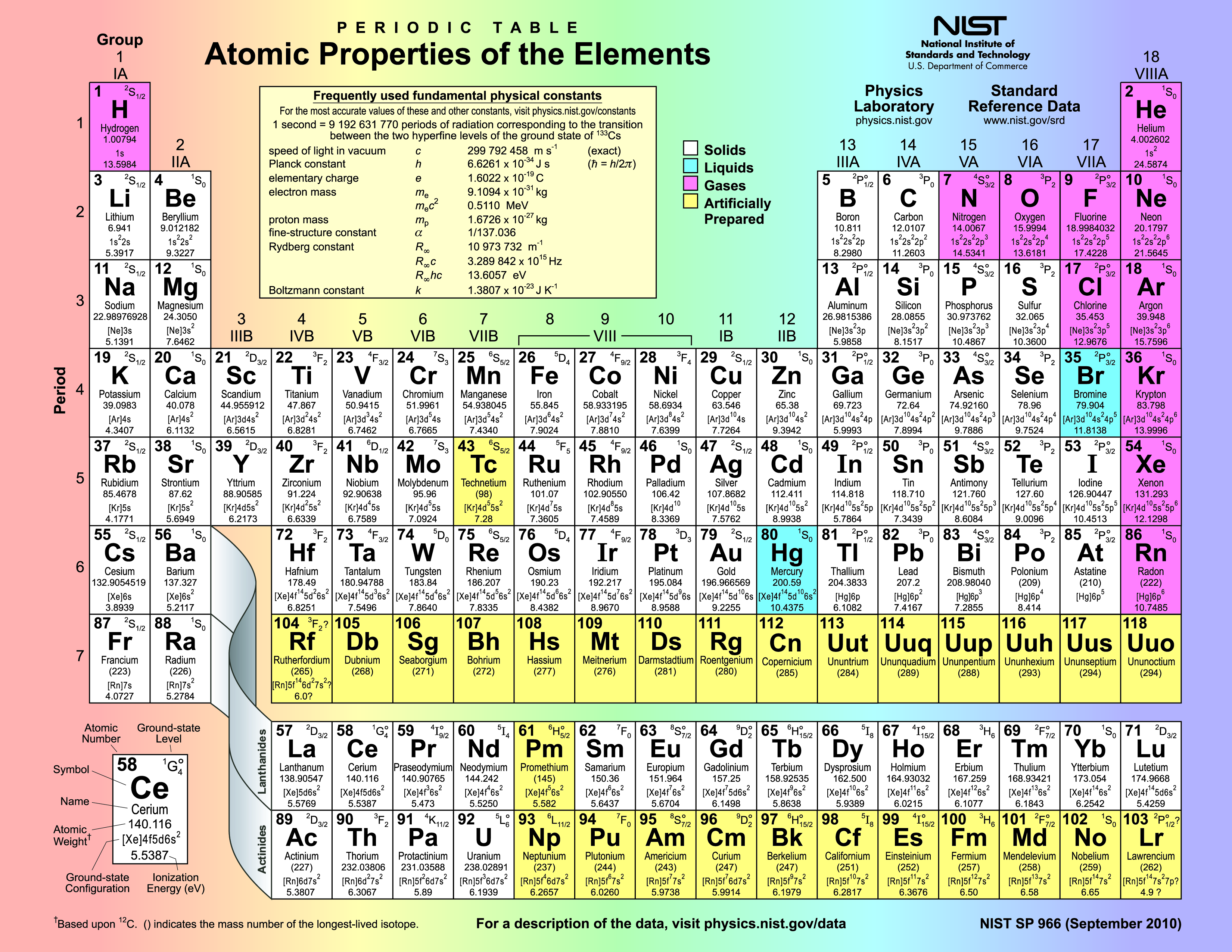
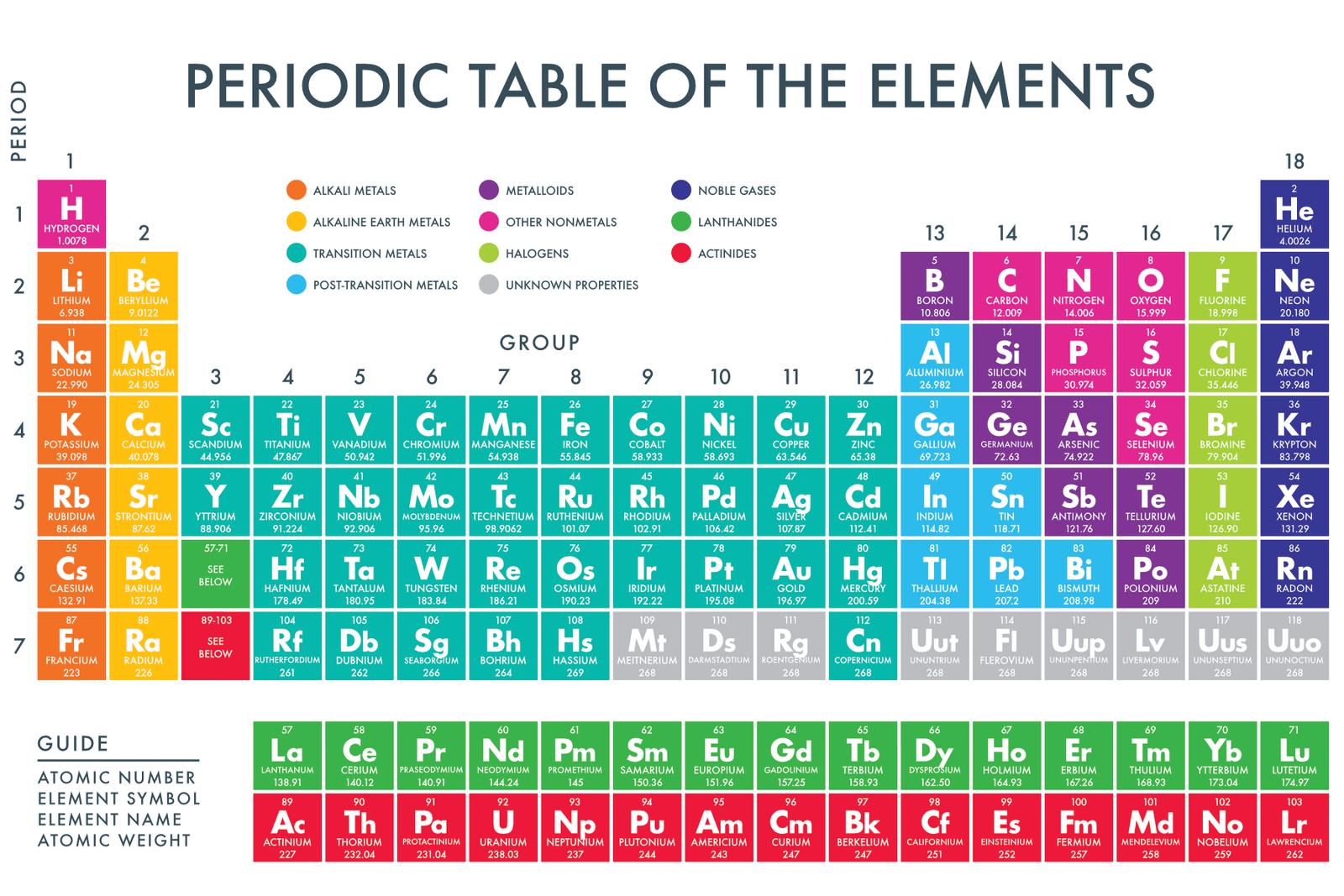
Alright, gather 'round, folks! Let's talk about those flashy characters on the periodic table – the transition metals. You know, the ones that aren't quite as predictable as your grandma's meatloaf recipe. We're not talking about your basic alkali metals, who are always eager to donate an electron like it's Black Friday. No, the transition metals are a whole different ball game.
So, where do we find these enigmatic elements? Imagine the periodic table as a bustling city. The alkali metals and alkaline earth metals? They're like the friendly, predictable folks in the suburbs, always on the left. The halogens and noble gases? They're the cool kids hanging out on the far right, all mysterious and aloof. And then, right smack-dab in the middle of all the action, wedged between the super-eager and the super-chill, you'll find our transition metal friends. Think of them as the downtown core, the heart of the periodic table party.
The "d-block" Dance Floor
More technically, the transition metals occupy what chemists affectionately call the "d-block." I know, it sounds like a particularly boring section of your local library, but trust me, it's where the magic happens. The d-block consists of groups 3 through 12 on the periodic table. That's a huge swathe of elements, and each one is unique in its own slightly quirky way.
Think of it like this: each row represents a new energy level. In the d-block, the electrons start filling the d orbitals, which are shaped like…well, let’s just say they're complicated and a little bit like electron-shaped pretzels. These d orbitals are what give transition metals their unique properties.
Why are they called "transition" metals? Well, it's because they represent a transition between the highly reactive s-block elements (like sodium and potassium) and the less reactive p-block elements (like carbon and oxygen). They're bridging the gap, bringing people – err, elements – together!
Here's a fun fact: the reason they're so good at forming colorful compounds is because those d orbitals can absorb different wavelengths of light. Think of it like a built-in disco ball. Copper compounds are often blue or green, chromium compounds can be vibrant green, and manganese can give you purple. Pretty cool, right?
Lanthanides and Actinides: The "f-block" Side Show
Now, just when you thought you had it all figured out, there's a plot twist! Below the main body of the periodic table, there are two rows of elements called the lanthanides and actinides. These guys are technically considered "inner transition metals" and are part of the "f-block." We had to make it more confusing, didn’t we?
Why are they down there, separated from the cool kids in the d-block? Well, it's all about keeping the periodic table from getting ridiculously wide. Imagine if we tried to squeeze them into the main table – it would stretch across the room! So, we tuck them away neatly below, like a secret underground club.
The f-block elements are even more exotic than the d-block. Their electrons start filling the f orbitals, which are even more pretzel-shaped than the d orbitals. The lanthanides (also known as the rare earth elements) are used in all sorts of high-tech applications, from smartphones to MRI machines. The actinides, on the other hand, are mostly radioactive, and some of them are downright scary. Uranium, for example, is used in nuclear power plants and...well, you know.
Properties that Pop
So, what makes transition metals so special? They're usually hard, strong, and have high melting and boiling points. They're also excellent conductors of electricity and heat, which is why copper is used in wiring and cookware. They're also incredibly versatile, able to form a wide range of compounds with different oxidation states. Basically, they can be whatever they want to be!
Many transition metals are also essential for life. Iron, for example, is a key component of hemoglobin in our blood, which carries oxygen from our lungs to the rest of our bodies. Zinc is important for our immune system, and copper is involved in various enzymatic processes. So, next time you're feeling tired or run down, maybe you just need a little more transition metal in your diet (consult your doctor first, of course!).
In conclusion, the transition metals, found in the d-block and f-block of the periodic table, are a fascinating group of elements with a wide range of properties and applications. They're the hard-working, colorful, and versatile players in the periodic table game. So, next time you look at the periodic table, give a little nod to these unsung heroes. They deserve it!