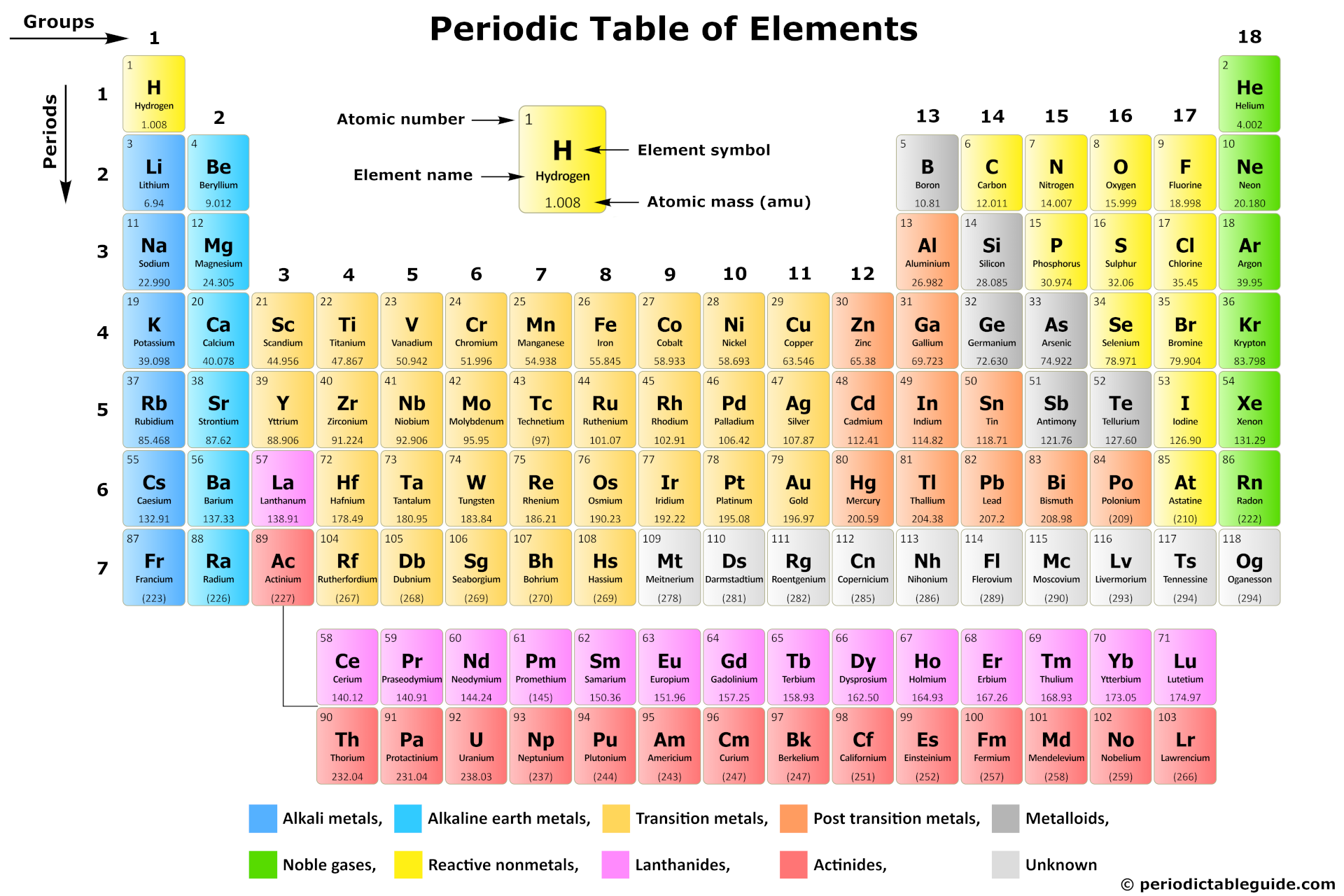
Hey there, chemistry curious! Ever stared at the periodic table and felt a slight... bewilderment? Don't worry, you're not alone! Let's zoom in on a particularly interesting bunch: the transition metals.
Think of the periodic table as a neighborhood. You've got your alkali metals, your halogens... and then there's this whole block of, well, metal in the middle. That's where our transition metal friends hang out!
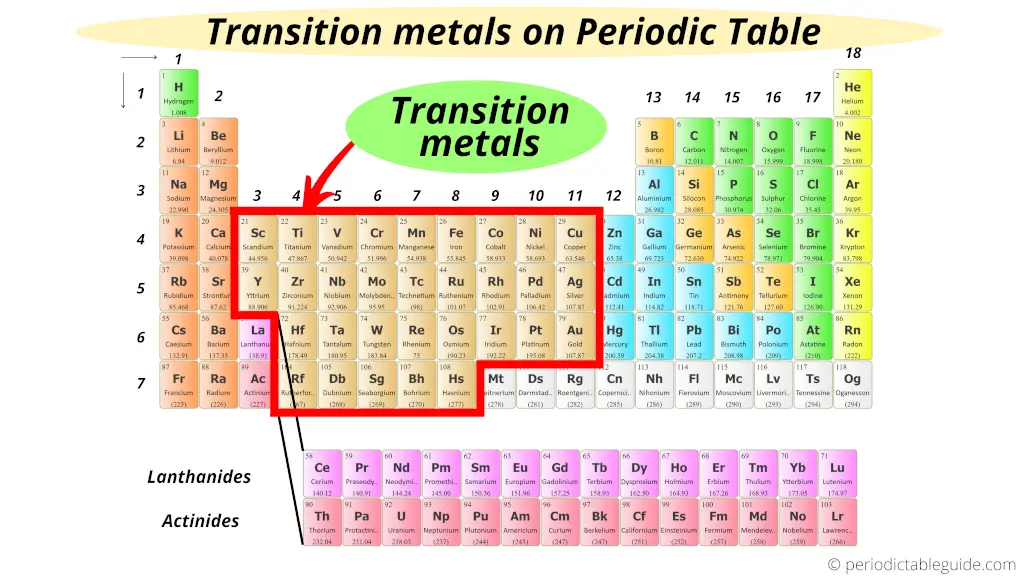
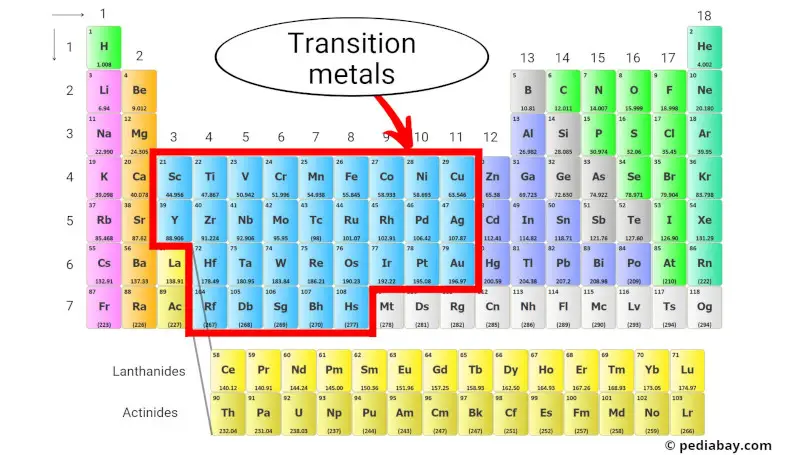
The Periodic Table's Cool Middle Kids
Specifically, look at groups 3 through 12. See that big chunk between the super reactive alkali metals (group 1) and the metalloids and nonmetals? Bam! Transition metals central.
They're like the cool kids in the periodic table cafeteria. Why? Because they're involved in *so much* stuff. They're the workhorses of the element world. Think strong, versatile, and often brightly colored.
Remember that time you saw a shiny gold ring? Gold is a transition metal! What about that strong, rust-resistant stainless steel pot in your kitchen? Thank iron, another transition metal, and its buddies for that!
Why Are They *There*?
Okay, science time (but I promise, it won’t hurt!). The periodic table is organized based on electron configurations, which basically means how the electrons are arranged around the atom. Transition metals are special because their electrons are filling what's called the "d orbital."
Think of orbitals like floors in an apartment building. Some floors are easier to fill than others. The d orbital is a bit quirky and allows transition metals to have multiple oxidation states – meaning they can form bonds in a variety of ways.
This flexibility is why they can make so many different compounds and why they’re so useful as catalysts (things that speed up chemical reactions). Imagine them as tiny matchmakers for molecules!
Colorful Personalities (Literally!)
One of the coolest things about transition metals? Their colors! Remember that blue copper sulfate you might have seen in a science experiment? Or the green emeralds? Those vibrant hues often come from the way transition metals interact with light.
The varying oxidation states mean that the color changes depending on what the metal is bonded to. It's like they're wearing different outfits depending on their mood! For example, vanadium can be bright purple, blue, green, yellow, or even colorless, depending on its oxidation state.
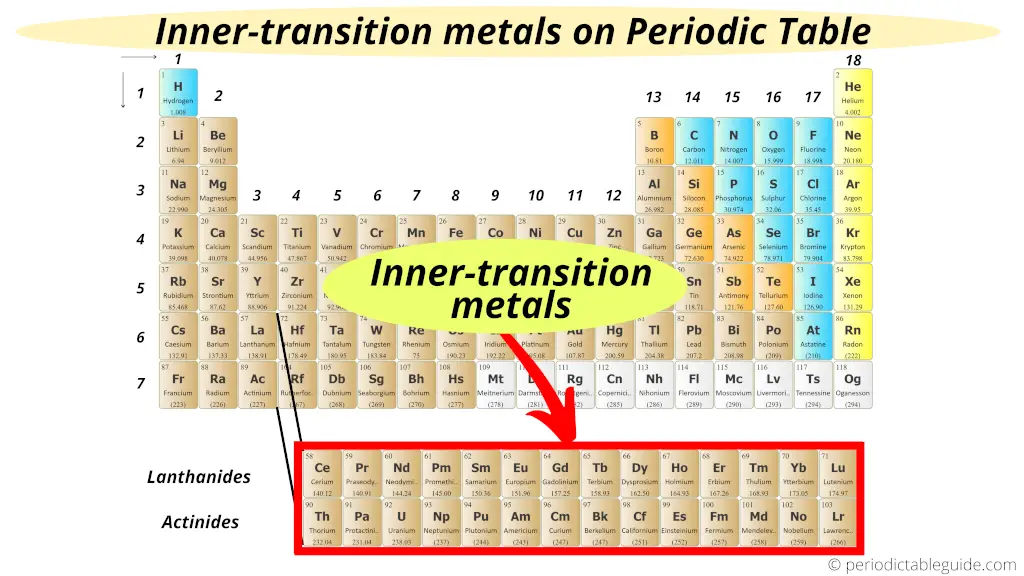
Lanthanides and Actinides: The Detached Family
Now, there's a bit of a "family secret" on the periodic table. See those two rows pulled out at the bottom? Those are the lanthanides and actinides. They're *technically* transition metals too! They're just so similar in their properties that they got their own designated spot.
Think of them as the cousins who live in a far-off land. They're still part of the family, but they have their own unique traditions (like radioactivity for some of the actinides!).
Why Should *You* Care?
Okay, so you might not be planning to become a chemist, but understanding where transition metals are on the periodic table is surprisingly useful!
They're in our batteries, our pigments, our electronics, our jewelry... Basically, they're everywhere! Knowing their location on the periodic table helps us understand their properties and how they're used in the world around us.
Plus, it's just cool to know that the colorful, strong, and versatile elements in the middle of the periodic table aren't just randomly placed. There's a reason they're there, and it's all thanks to the fascinating world of electron configurations!
So, next time you glance at the periodic table, give a little nod to the transition metals. They're the unsung heroes of the element world, quietly making our lives brighter, stronger, and a whole lot more colorful! Keep exploring!