Hey there, chemistry curious minds! Ever gazed at the periodic table, that colorful grid of elements, and wondered about that chunky block smack-dab in the middle? Yeah, I'm talking about the transition elements. What's their story? Where do they fit in the grand scheme of chemical things? Let's dive in and find out!
Finding the Treasure: Location, Location, Location!
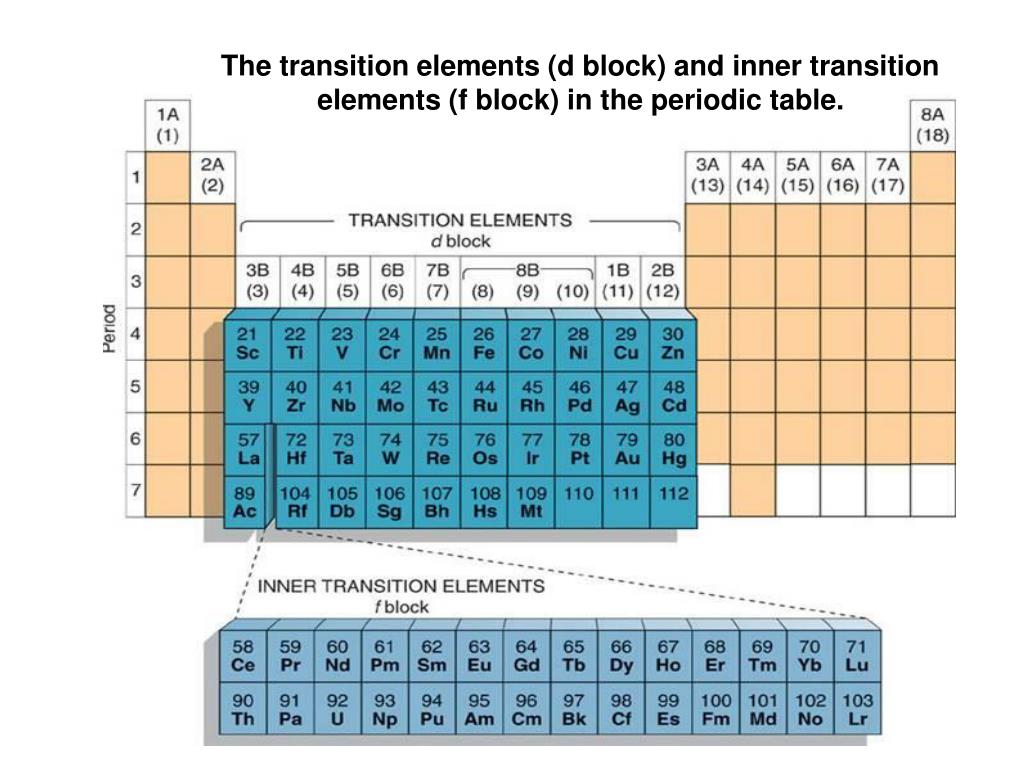
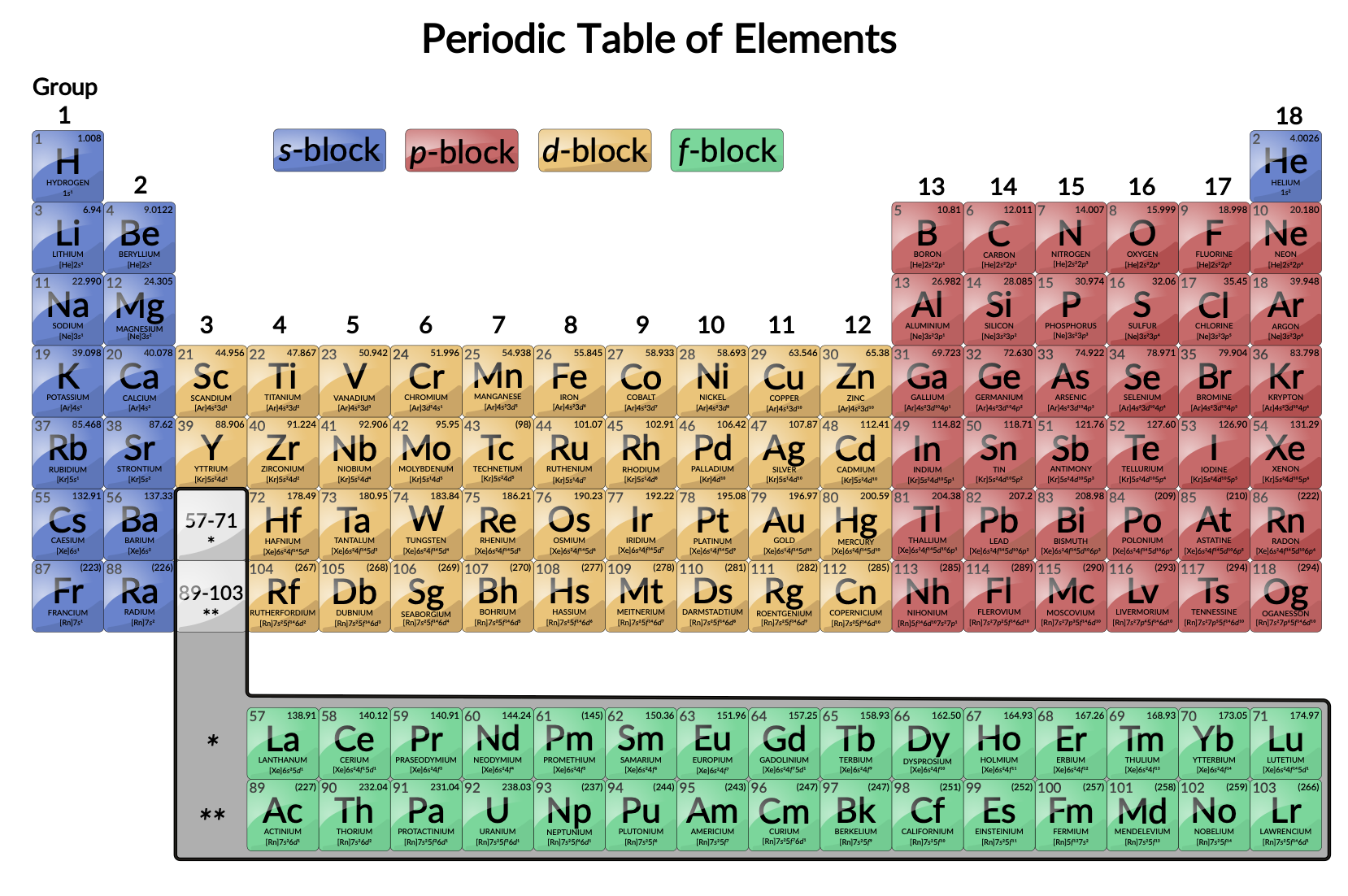
Okay, so where exactly are these transition elements hanging out? Easy peasy. Look at your periodic table. Ignore the super-light elements on the left (like hydrogen and lithium) and those eager-to-react ones on the right (like oxygen and chlorine). See that big, rectangular block sitting right between groups 2 and 13? That's transition metal territory!
Think of it like this: the periodic table is a city. The alkali metals on the far left are the trendy, always-out-and-about folks. The noble gases on the far right? They're the reclusive celebrities, rarely seen interacting. The transition metals? They're the bustling downtown, the heart of the action, where all sorts of interesting business goes down.
More specifically, the transition elements occupy the d-block of the periodic table. What's a d-block, you ask? Don't worry about the details too much right now, but basically, it has to do with how their electrons are arranged. These elements are filling their 'd' orbitals with electrons, which gives them some pretty unique properties. Fancy, right?
Why the Middle Matters: Cool Properties and Hidden Talents
So, they're in the middle... big deal, right? Wrong! Their location is actually key to understanding why they're so cool. Because of their electron arrangements, transition elements are responsible for a ton of cool stuff you see around you every day.
Ever wondered where vibrant colors in paints, gemstones, and stained glass come from? Often, it's the work of transition metal ions! The electrons in these metals can absorb and emit light at specific wavelengths, creating those beautiful hues. Think of copper's blue-green patina, the rich reds of iron oxide (rust!), or the dazzling purple of amethyst (which gets its color from iron impurities). Pretty neat, huh?
But wait, there's more! Many transition metals are fantastic catalysts. What's a catalyst? It's like a chemical matchmaker, speeding up reactions without being used up itself. Platinum, for example, is used in catalytic converters in cars to reduce harmful emissions. Iron is a crucial catalyst in the Haber-Bosch process, which produces ammonia for fertilizers, helping to feed the world! These metals are real workhorses.
And let's not forget their strength! Transition metals are generally strong, durable, and have high melting points. Iron, nickel, chromium, and titanium are used to create strong alloys for building structures, airplanes, and... well, just about everything! Can you imagine a world without steel (mostly iron)? That's a world without skyscrapers, bridges, or even spoons!
Families within the Family: Exploring the Groups
Now, just like any big city, the transition metal block has different neighborhoods – or, in periodic table lingo, different groups. Each group shares some similarities in their chemical behavior. Think of it like families: they might look a little different, but they share some core traits.
For example, the iron triad (iron, cobalt, and nickel) are known for their magnetic properties. The platinum group metals (ruthenium, rhodium, palladium, osmium, iridium, and platinum) are incredibly rare and valuable, often used in jewelry and electronics.
Don't forget about the lanthanides and actinides, those two rows chilling at the bottom of the periodic table! They're technically inner transition metals, meaning they're filling 'f' orbitals instead of 'd' orbitals. They're often used in nuclear technology and high-tech applications. Think of them as the super-specialized, high-performance division of the transition metal world.
So, What's the Big Deal?
Why should you care about where the transition elements are located on the periodic table? Because their location isn't just some random placement. It dictates their electronic structure, which in turn dictates their properties, and ultimately, their usefulness in our world. From the vibrant colors in your art supplies to the strong steel in your buildings, transition metals are essential ingredients in the recipe of modern life.
Next time you glance at the periodic table, take a moment to appreciate that bustling downtown of transition elements. They're more than just a block of metals; they're the key to unlocking a world of fascinating chemistry and amazing applications. So, keep exploring, keep questioning, and keep being curious!