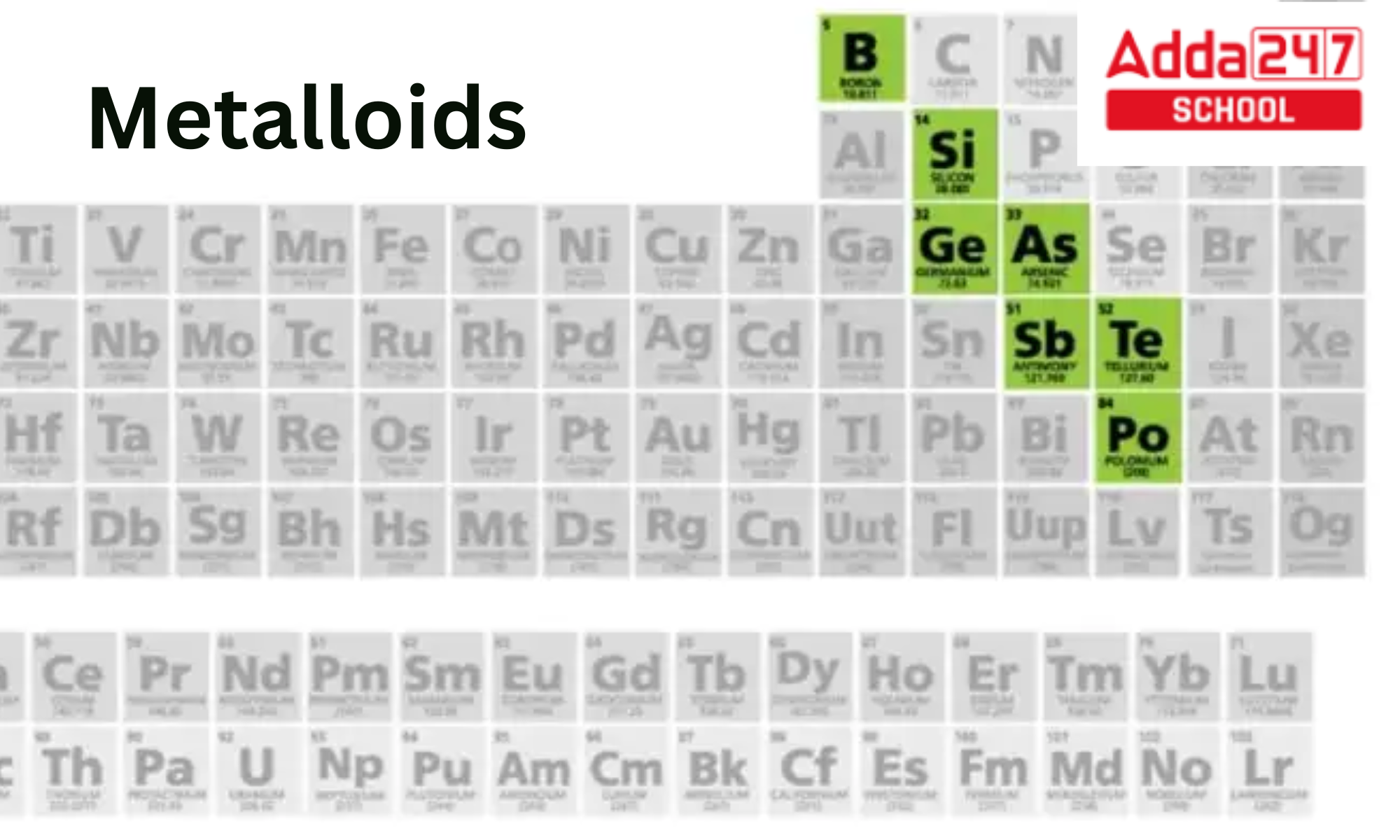
Ever wonder where to find that quirky middle ground on the periodic table? The place where elements can't quite decide if they're metallic rock stars or non-metallic wallflowers? Well, that's the realm of the metalloids! They might not be as famous as gold or as ubiquitous as oxygen, but these elements hold a special appeal, especially for those of us who love to tinker, create, and simply learn.
For artists and hobbyists, understanding metalloids can unlock a new level of creativity. Think about the stunning colors in stained glass. Often, these vibrant hues are thanks to metalloid compounds. Or consider the world of electronics – from the circuits in your phone to the solar panels on your roof. Metalloids like silicon and germanium are absolutely essential! Knowing their properties can inspire new projects, from crafting unique jewelry with semi-conductive elements to understanding the science behind your favorite gadgets.
Casual learners will also find metalloids fascinating. They're a perfect example of how the world isn't always black and white. They exist in a gray area, exhibiting properties of both metals and nonmetals. It's like having a secret key to understanding the nuances of chemistry!
So, where exactly are these enigmatic elements hiding on the periodic table? They form a sort of diagonal staircase. If you look at a periodic table, you'll usually find them clustered along a line that starts near boron (B) and goes down and to the right, including elements like silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te). Sometimes polonium (Po) and astatine (At) are also included, depending on the source.
Want to bring the magic of metalloids home? You probably already have! Silicon is in the sand in your backyard (though you can't just melt it down and make computer chips, sadly!). You can explore how different types of glass (which contain silicon) react to light. Or, investigate the conductivity of different materials using a simple circuit with a battery, a lightbulb, and some wires. Try testing different "metalloid-adjacent" objects, like a graphite pencil lead (mostly carbon, a nonmetal near the metalloids) versus a copper wire.
Don't forget the visual side. Look at images of crystalline silicon – it's stunning! Find pictures of different arsenic compounds; some are beautifully colored. Research how germanium is used in infrared optics. Just diving into the visual representation of these elements and their uses can be a creative journey in itself.
The best part about exploring metalloids is that it's accessible to everyone. You don't need a fancy lab or a chemistry degree. It's about curiosity, observation, and a little bit of playful experimentation. And who knows? You might just discover a new passion or a newfound appreciation for the amazing building blocks that make up our world. So, go ahead, embrace the gray area! Explore the metalloids. You'll find they're a lot more exciting than you might think. Happy exploring!