Hey there! Grab a mug, settle in. We're gonna chat about coal, right? Not exactly the most glamorous topic, I know, but trust me, there's some cool stuff tucked away in those dusty black lumps. Ever wondered, like, really wondered, what type of energy coal actually is?
Because, let's be honest, it just looks like a rock. A very dirty, burnable rock, sure, but still. It doesn't exactly scream "power source" until you, you know, light it on fire. Which, spoiler alert, is exactly what we do!
So, What's the Big Secret?
Alright, no more suspense. At its heart, coal is a form of chemical energy. Yep, that's the big one. It's energy stored up in the bonds between atoms and molecules. Think of it like a tiny, invisible battery, just waiting for a reason to discharge.
But that's not the whole story, not by a long shot. Because where did that chemical energy even come from in the first place? Did it just... poof... into existence? Nah, definitely not.
A Journey Through Time (and Sunlight!)
To really get it, we need to take a little trip back, like, millions of years. We're talking about the Carboniferous Period here – a time when Earth was basically one giant, swampy, super-lush jungle. Giant ferns, weird ancient trees, all that jazz.
Those plants, bless their ancient, leafy hearts, were doing what plants do best: photosynthesis. Remember that from school? They were soaking up sunshine, drinking water, and pulling carbon dioxide from the air. Basically, they were converting the sun's glorious light energy into their own sugary food. That food? That's chemical energy, right there.
So, those plants grew, they lived their best prehistoric lives, and eventually... they died. Sad, but essential for our story!
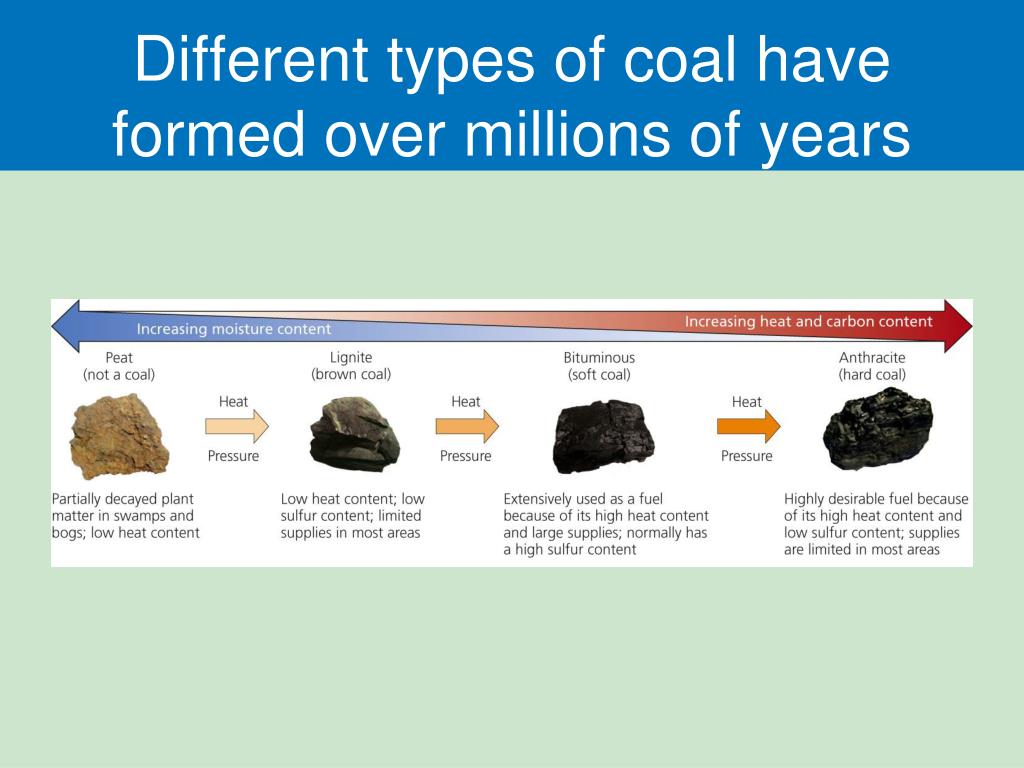
The Ultimate Burial & Transformation
Instead of just rotting away like normal leaves in your compost bin, these plants got buried. And I mean really buried. Under layers and layers of mud, sand, water, and other dead plant matter. It was like a giant, geological lasagna, but with much less cheese and way more pressure.
Over millions and millions of years, that immense pressure and heat from being squished deep underground worked their magic. The water got squeezed out, the volatile stuff evaporated, and what was left was mostly carbon. That's coal, folks! A fossilized record of ancient sunlight, transformed into a dense, energy-rich rock.
So, while it's fundamentally chemical energy, it's really the ultimate expression of stored solar energy. How cool is that? The sun's power, bottled up in a rock, waiting for a chance to shine (or, you know, burn).
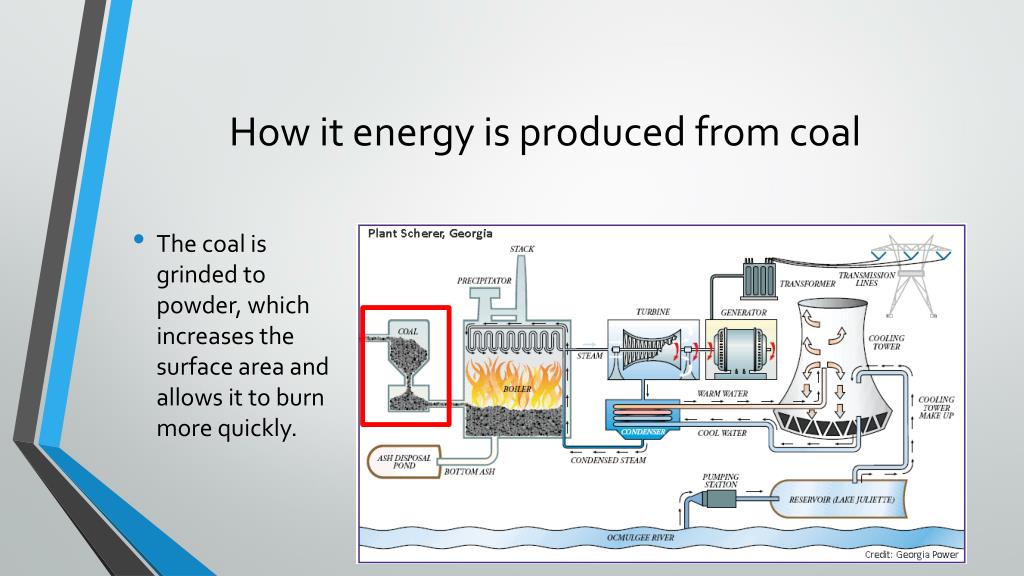
When We Burn It, What Happens?
Okay, so we've got this chemically-charged, ancient-sun-powered rock. What happens when we toss it into a furnace, say, at a power plant?
We're essentially breaking those chemical bonds that have held that stored energy for eons. And when those bonds break, guess what? They release energy! Most of that energy comes out as heat and light.
That heat then gets to work, usually boiling water to create steam. That steam spins giant turbines, which then power generators, and voilà! Electricity for your phone, your lights, your Netflix binge. It's a whole chain reaction, all starting with those tiny, patient chemical bonds.
Is It Like... Food?
Kind of! Think about the food you eat. A burger, a banana, a delicious donut – they all contain chemical energy that your body breaks down to power *you*. Coal is doing a similar thing, just on a much grander, industrial scale, and it's powering, well, everything else!
So, the next time you hear about coal, remember it's not just a lump of rock. It's a geological marvel, a time capsule of Earth's ancient past, and a powerful, albeit often controversial, source of chemical energy, ultimately thanks to the sun. Pretty neat, right? Who knew dirt could be so fascinating?