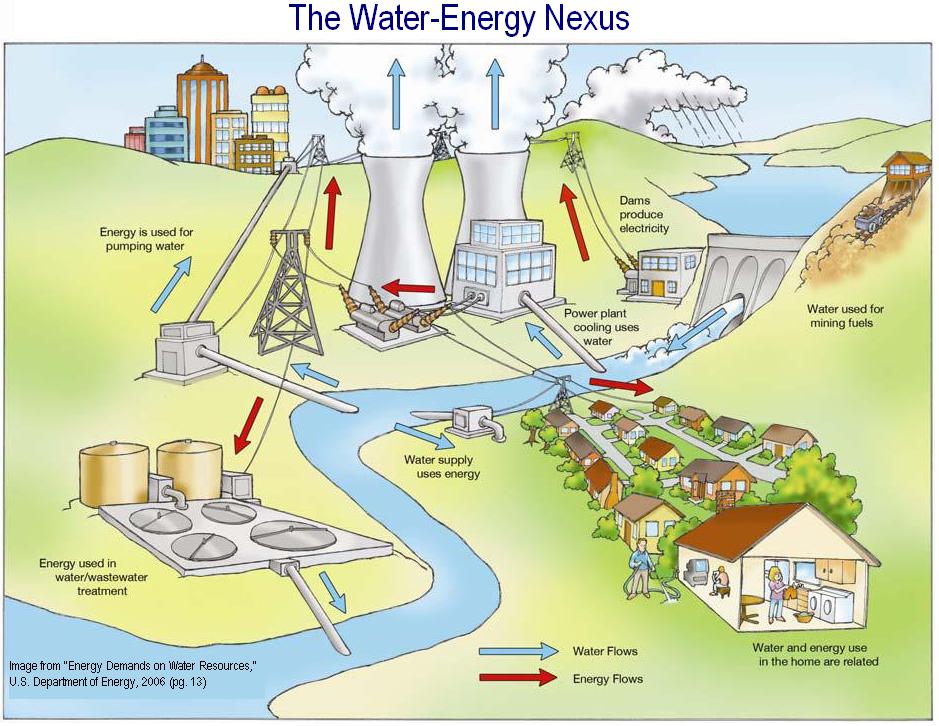
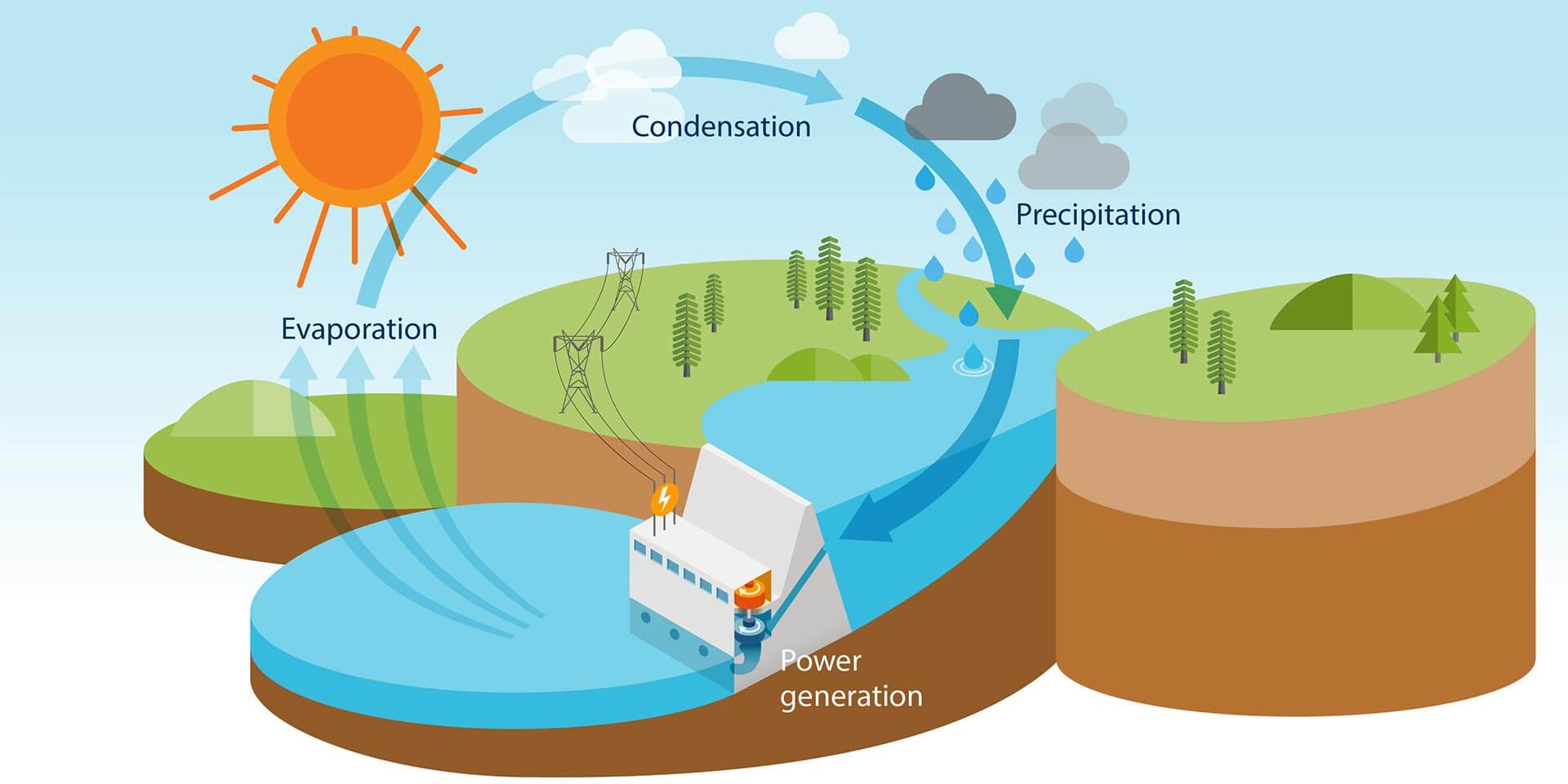
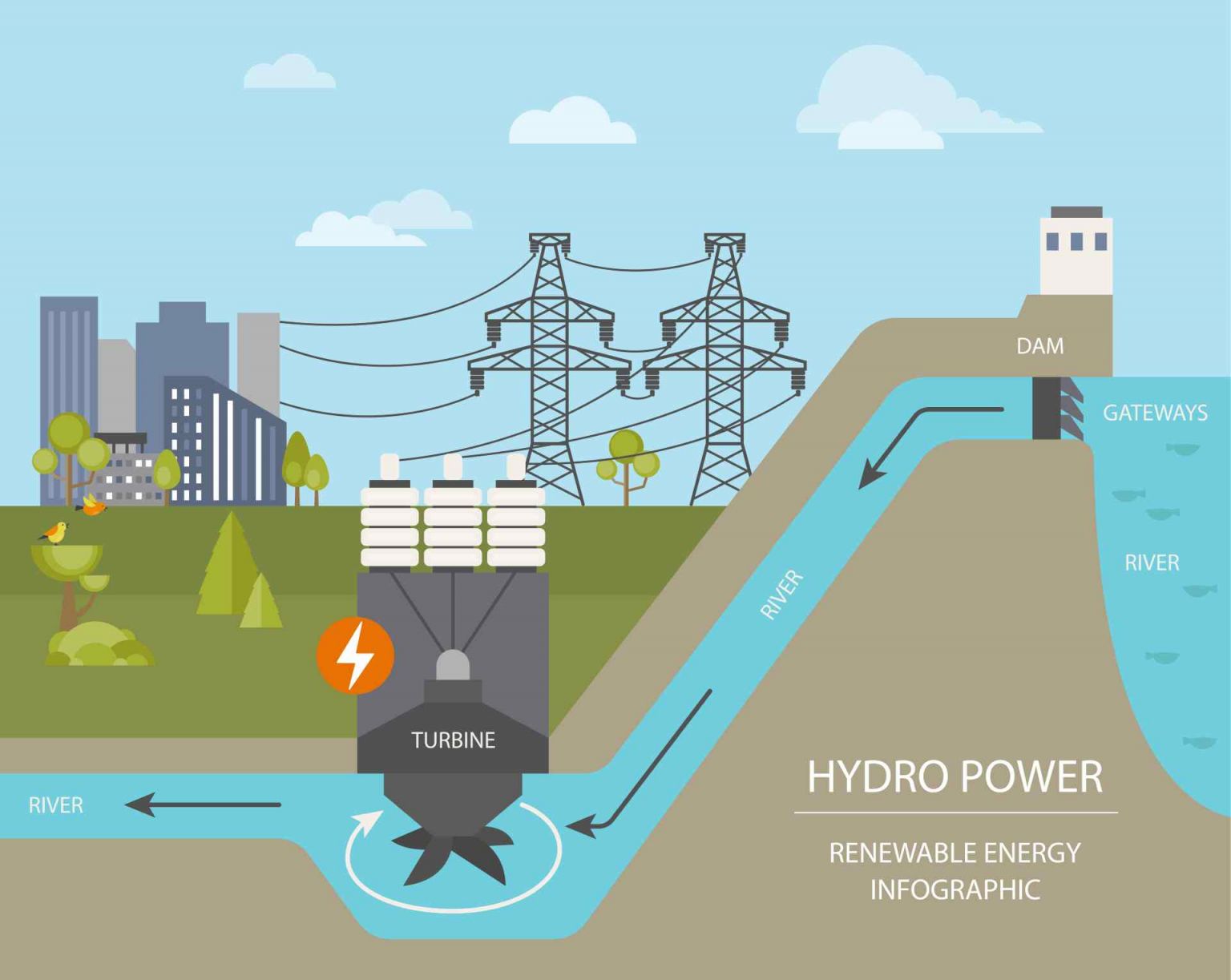
Ever filled up a glass of water, taken a refreshing gulp, and then thought, "Could this... power my house?" It's a pretty wild question, right? We drink it, swim in it, wash with it – but is water actually a *source of energy*? Let's dive in and get a little curious about one of Earth's most amazing compounds!
At first glance, the answer might seem like a straightforward "no." You can't just pour water into your car's fuel tank and expect it to go (please don't try that!). Water isn't flammable like gasoline, and it definitely doesn't store energy in the same way a battery does. So, if we're talking about burning it or using it as a direct chemical fuel, then nope, water isn't your go-to.
But Wait, There's a Ripple!
Ah, but the world of energy is rarely that simple, is it? While you can't *burn* water, water is absolutely essential to some of the biggest and oldest forms of energy generation on our planet. Think about it:
Hydropower! This is probably the first thing that comes to mind for many. When you see a massive dam holding back a huge reservoir, you're looking at a giant battery of potential energy. That water, held high, has immense gravitational pull. When it's released and rushes downhill, it spins turbines, which then power generators. Voila! Electricity!
It's like a cosmic waterslide for energy! The water itself isn't *creating* the energy; it's the *movement* and *fall* of the water that converts its potential energy (stored by being high up) into kinetic energy (energy of motion), and then into electricity. It’s incredibly clean and efficient, harnessing nature's own power. And let's not forget about tidal power or wave power, which use the ocean's consistent rhythms to generate electricity. Again, it’s all about harnessing that lovely, powerful *motion* of water.
The Hydrogen Hype: Splitting H₂O
Now, things get a little more sci-fi, but still super cool. You might have heard about "hydrogen fuel cells" and the idea of a "hydrogen economy." Water is made of two hydrogen atoms and one oxygen atom (H₂O). What if we could split that water molecule apart?
We totally can! It's called electrolysis, and it uses electricity to break those chemical bonds, separating the hydrogen from the oxygen. Once you have pure hydrogen, it *can* be used as a fantastic fuel. Burn it, and it releases a lot of energy, with the only byproduct being... you guessed it, water! Talk about a neat, closed loop.
So, if hydrogen comes from water and can be a fuel, does that mean water is an energy source? Well, here's the tricky part, and it's a bit like trying to lift yourself by your bootstraps.
To split water into hydrogen and oxygen, you have to put energy in. And generally, that energy input is *more* than the energy you'll get back out when you burn the hydrogen. It's not magic! It's more like using energy to *store* energy in a different form. Think of it like charging a phone battery – you put electricity in, and then you can use that stored energy later. The phone battery isn't a "source" of energy; it's a carrier or storage device.
The real game-changer for hydrogen is when the energy we use for electrolysis comes from renewable sources like solar or wind power. Then, you're essentially using sunlight or wind to create a clean-burning fuel (hydrogen) from water, which can then be stored and used when the sun isn't shining or the wind isn't blowing. That's super exciting!
Other Aquatic Wonders
There are even more ingenious ways scientists are trying to squeeze energy out of our watery world. Have you heard of Ocean Thermal Energy Conversion (OTEC)? This ambitious idea uses the temperature difference between warm surface water and cold deep-ocean water to drive a heat engine and generate electricity. It’s incredibly complex, but the potential is mind-boggling, given how much of our planet is covered by oceans!
The Verdict: A Facilitator, Not a Fuel
So, is water a direct source of energy? Not in the way a lump of coal or a barrel of oil is. You can't just tap into its intrinsic chemical makeup and release a surge of power for free. Water is stable, which is why it's so perfect for life!
However, water is an absolutely crucial medium and carrier for energy. It's the powerful current in hydropower, the raw material for future hydrogen fuels, and the vast reservoir of thermal differences in OTEC. It’s a facilitator, a conduit, and a storage system for some of the planet's most promising clean energy solutions.
Next time you look at a glass of water, maybe you'll see more than just a refreshing drink. You might see a tiny glimpse into the future of clean energy, a powerful force of nature, and a testament to human ingenuity. Pretty cool for something so simple, right? The more we understand water, the more amazing it becomes!