So, picture this: You wake up, maybe you hit the snooze button one too many times (guilty as charged!), stumble to the coffee machine, flick on the light switch. Later, you hop in your car, turn the key, and off you go. Easy, right? Every day, we use an incredible amount of energy, from charging our phones to flying across continents. But have you ever, *really* ever, stopped to ponder where all that juice, all that get-up-and-go for your car, your lights, your everything, actually comes from? I mean, beyond the gas station pump or the power plant, obviously. Because, trust me, it’s a story way cooler than just a tanker truck.
It’s not some wizard conjuring energy out of thin air, though sometimes it feels that magical, doesn't it? No, the truth is even more epic. We're talking about a process that spans millions of years, involves microscopic life, dead forests, and a whole lot of squeezing and cooking deep underground. Pretty wild, right?
The Original Ingredients: Life, Death, and Time
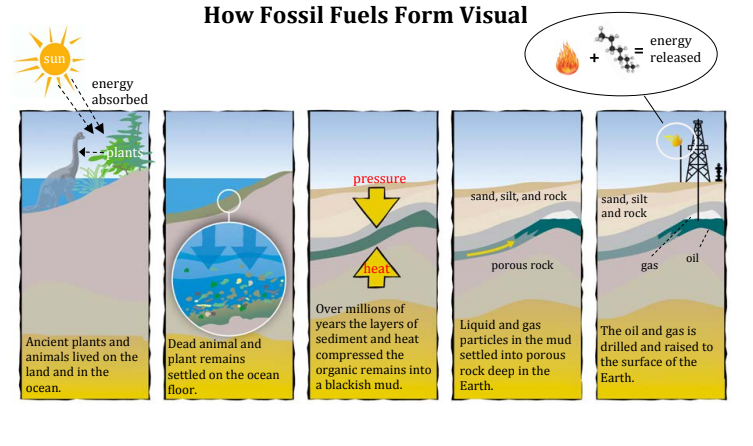
At its heart, fossil fuel creation starts with, well, life. Specifically, massive amounts of organic matter. We’re talking about ancient plants and animals that lived eons ago.
For coal, think vast, swampy forests. Imagine lush, giant ferns and trees from the Carboniferous period (that's roughly 300 million years ago, give or take a few million!). When these plants died, they didn't just decompose fully like they usually do today. Instead, they piled up in those waterlogged swamps. Why? Because the water prevented them from fully rotting away due to a lack of oxygen. This created thick layers of partially decayed plant matter called peat.
Now, for oil and natural gas, shift your mental image from swamps to ancient oceans and shallow seas. Here, the primary "ingredients" were tiny marine organisms – think algae, plankton, and other microscopic critters. When these fellas died, their remains drifted to the seabed. Again, in areas with low oxygen (anaerobic conditions), they didn't fully decompose. Instead, they got preserved.
The Underground Kitchen: Heat, Pressure, and a *Really* Long Wait
So, you’ve got these layers of organic goo (peat for coal, marine muck for oil/gas). What happens next is where the real transformation begins. Over unimaginable stretches of time – we're talking not just thousands, but millions of years, folks! – more and more sediment started piling on top. Think layers of mud, sand, and rock.
This constant piling up meant two things: immense pressure from the weight of all those overlying layers, and increasing heat as these organic layers got buried deeper and deeper into the Earth's crust. It’s like the Earth itself is a giant, super-slow-cooker pressure cooker.
Under this incredible heat and pressure, the organic matter started to change chemically.
- For the peat (from the swamps), it gradually lost water and other compounds, becoming denser and more carbon-rich. First it became lignite, then sub-bituminous coal, then bituminous coal, and if it got *really* squeezed and heated, even anthracite – the hardest and most energy-dense form of coal. Voilà: coal!
- For the marine organisms (from the oceans), the process was similar. The organic goo, often called kerogen at this stage, was literally cooked. The heat and pressure broke down these complex organic molecules into simpler hydrocarbon chains. These lighter hydrocarbons form liquid oil and natural gas. This usually happens at specific temperature and pressure "windows" – too little, and you get kerogen; too much, and you might just get graphite!
The Great Migration (for Oil and Gas)
Once the oil and natural gas are formed in what we call the "source rock" (that original layer of cooked marine sediment), they don't necessarily stay put. Because they're lighter than the surrounding rock and water, they often begin to migrate upwards, seeping through tiny pores and cracks in the rock.
They keep moving until they hit a layer of impenetrable rock, called a "cap rock." This cap rock acts like a lid, trapping the oil and gas in what's known as a "reservoir rock" – typically a porous rock like sandstone. And *that's* where we find those huge underground pools and pockets of petroleum that we drill into today. Pretty clever, nature, wouldn't you say?
The Takeaway: Ancient Sunlight, Today's Power
So, the next time you flick a switch or fill up your tank, remember that you’re not just using a commodity. You’re tapping into sunlight that was captured by plants and tiny organisms hundreds of millions of years ago. You're using energy that has been cooked, squeezed, and preserved deep within the Earth's crust over geological timescales.
It's a process that's almost unfathomable in its duration and scale. It's a testament to the incredible power of time, geology, and the persistent cycle of life and death on our planet. Mind-blowing, isn't it? And all this, just so we can binge-watch our favorite shows or grab a latte without breaking a sweat!