Hey there! So, you're wondering about water and its whole "heat capacity" thing? Let's dive in, shall we? (Pun absolutely intended.)
Okay, so what even is heat capacity? Imagine trying to heat up a tiny metal spoon versus, say, a giant swimming pool. The spoon gets hot super fast, right? But the pool? It takes forever. That's basically heat capacity in action! It's how much energy something needs to absorb to change its temperature.
So, does water have a high heat capacity? You bet your bottom dollar it does! It's actually kinda famous for it. We're talking really high. Like, Olympic athlete level high. Okay, maybe I'm exaggerating a little. But seriously, it's up there.
Why is this such a big deal, you ask? Well, think about it. If water heated up super easily, like that metal spoon, things would be... chaotic, to say the least. Our oceans would boil during the day and freeze at night! Yikes! Glad that's not the reality, huh?
That high heat capacity means water can absorb a lot of heat without drastically changing its temperature. It's like a thermal sponge, soaking up all that energy from the sun. Pretty cool, right?
And it's not just oceans. Think about your body. We're mostly water, too! (About 60%, give or take, depending on who you ask and how much pizza you ate last night). If water didn't have that high heat capacity, we'd overheat just walking to the mailbox. Imagine needing an ice bath after checking your mail. No thanks!
So, what makes water so special? Good question! It's all about those tiny little water molecules and how they like to hang out together. They're held together by these things called hydrogen bonds. (Don't worry, no need to get too scientific here.) These bonds are like little magnets that attract the water molecules to each other.

Breaking those bonds to get the water to heat up takes a lot of energy. That's why it needs so much heat to actually change its temperature. It's too busy dealing with its intermolecular relationships to worry about getting hot quickly!
Think of it like this: imagine trying to get a group of super-glued people to start running a race. You'd have to use a ton of energy to break them apart first, right? Same with water molecules and those pesky hydrogen bonds.

Let's recap, because why not? Water's high heat capacity means:
- Oceans don't boil during the day. A major plus, wouldn't you agree?
- Our bodies don't overheat from simple everyday tasks. Another win!
- It helps regulate Earth's climate. Super important for, you know, life on Earth.
So, next time you're swimming in the ocean, taking a shower, or just drinking a glass of water, remember that amazing high heat capacity. It's working hard to keep everything nice and stable. You could say water is a real team player!

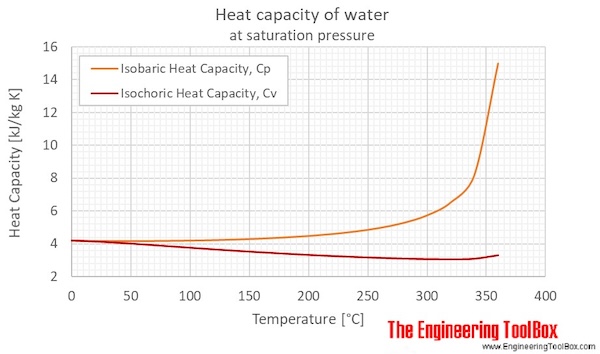
One more thing: Don't confuse heat capacity with specific heat. They're related, but not the same! Heat capacity is the amount of heat needed to raise the temperature of an object by 1 degree Celsius (or Kelvin, if you're feeling fancy). Specific heat is the amount of heat needed to raise the temperature of 1 gram of a substance by 1 degree Celsius. See the difference? It's all about whether you're talking about a whole thing or just a tiny bit of it.
Anyway, that's the gist of it. Water: high heat capacity champion! Hope this made sense and wasn't too boring. Now, where's that refill of coffee?
In conclusion, water = awesome. Case closed! (For now, anyway.)