Hey there, curious minds! Ever wondered what makes the world around us tick? (I mean, besides caffeine, of course!) We're diving into the fascinating world of chemistry, specifically the quirky realm of nonmetals! Now, before your eyes glaze over, trust me, this is way more interesting than it sounds. Think of it as unlocking a secret code to understanding… well, everything!
So, what exactly are these nonmetals we speak of? Basically, they're the rock stars of the periodic table, but instead of screaming guitars, they have… well, different properties. The opposite of metals, as you might guess!
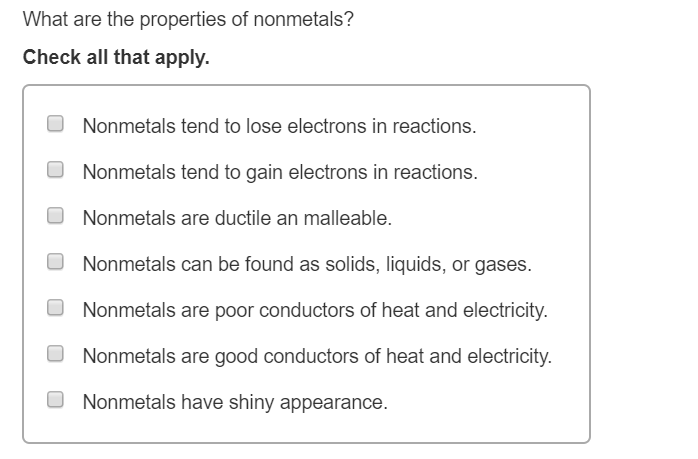
Which Properties Are Characteristics Of Nonmetals? Let's Investigate!
Okay, ready to play detective? We're going to go through a list of common characteristics and figure out which ones apply to our nonmetallic friends. Get ready to put on your thinking caps!
Poor Conductors of Electricity and Heat: Ding ding ding! We have a winner! Nonmetals are notorious for being terrible conductors. This means they don't let electricity or heat flow through them easily. Think of it this way: Would you use a rubber spatula to stir a boiling pot of soup? Probably not! Rubber, often made of nonmetals, is a poor conductor of heat, which is precisely why we use it for that purpose!
Dull Appearance: Another checkmark! Unlike shiny, shimmering metals, nonmetals tend to have a dull, lackluster appearance. No bling here, folks! Think of sulfur, a yellowish powder, or carbon in the form of graphite (pencil lead). Not exactly dazzling, right?
Exist in All Three States of Matter at Room Temperature: You bet! This is a biggie. Nonmetals are super versatile. You can find them as solids (like sulfur and carbon), liquids (like bromine), and gases (like oxygen, nitrogen, and chlorine) all at room temperature. Metals, on the other hand, are mostly solid. Talk about being adaptable!
Brittle or Gaseous: Generally! Many solid nonmetals are brittle, meaning they break easily when you try to bend or hammer them. They don't have that malleable (easily shaped) or ductile (easily drawn into wires) property that metals do. And, as we already mentioned, many nonmetals are gases at room temperature, like the air we breathe (oxygen and nitrogen). Important to add: Carbon fiber might seem very ductile, but it isn't a nonmetal in its pure form.
Low Melting and Boiling Points: Yes, indeed! Compared to metals, nonmetals generally have much lower melting and boiling points. This means it doesn't take as much heat to turn them into liquids or gases. Imagine melting ice (made of hydrogen and oxygen, both nonmetals) versus melting iron! Big difference!
Tend to Gain Electrons in Chemical Reactions: Absolutely! This is a key characteristic that differentiates nonmetals from metals. Nonmetals are more likely to *gain* electrons when they react with other elements, forming negative ions (anions). Metals, on the other hand, tend to *lose* electrons, forming positive ions (cations). It's all about sharing (or, in this case, taking!) electrons to achieve stability.
Why Should I Care About Nonmetals?
Okay, okay, I hear you. "Why should I care about this chemistry stuff?" Well, for starters, nonmetals are essential for life! Oxygen, nitrogen, carbon, hydrogen – these are all nonmetals, and they're the building blocks of everything that lives and breathes on this planet. Pretty important, right?
And beyond that, understanding the properties of nonmetals helps us develop new technologies and materials. From the plastics in our phones to the fertilizers that grow our food, nonmetals play a crucial role in modern society. Plus, knowing a little bit about chemistry is just plain cool! You can impress your friends with your knowledge of elements and reactions (or maybe just win at trivia night!).
Think about it: the air you breathe, the water you drink, the food you eat - all of these involve nonmetals and their unique properties. Understanding how they behave is like understanding the secrets of the universe. (Okay, maybe that's a little dramatic, but you get the idea!)
So, there you have it! A whirlwind tour of the wonderful world of nonmetals. I hope this has sparked your curiosity and made you realize that chemistry isn't just some boring subject you learned in high school. It's a fascinating and relevant field that helps us understand the world around us. Maybe now you feel the urge to learn more about the chemical compositions and reactions of your food.
Now go forth and explore the amazing world of science! There's so much to discover, and the more you learn, the more you'll appreciate the intricate beauty of the universe. The periodic table is waiting... are you ready to answer its call?