Hey there, curious minds! Ever wondered what keeps the world glued together? No, not just love (though that's important too!). We're talking about the super tiny stuff: atoms and their wild dance with energy. And guess what? We're about to dive into a super cool corner of that dance – ionization energy!
Now, I know what you might be thinking: "Ionization energy? Sounds like something from a sci-fi movie!" But trust me, it's way more relatable (and way less likely to involve aliens, probably). Think of it this way: every atom is like a tiny little fortress, holding onto its electrons. Ionization energy is basically how much "oomph" it takes to steal one of those electrons.
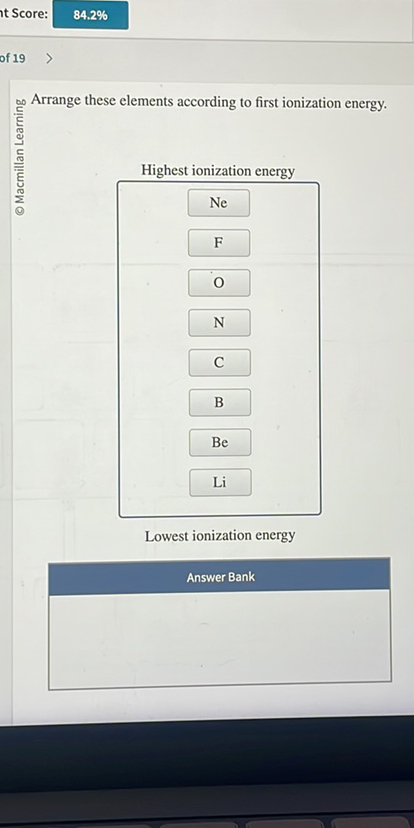
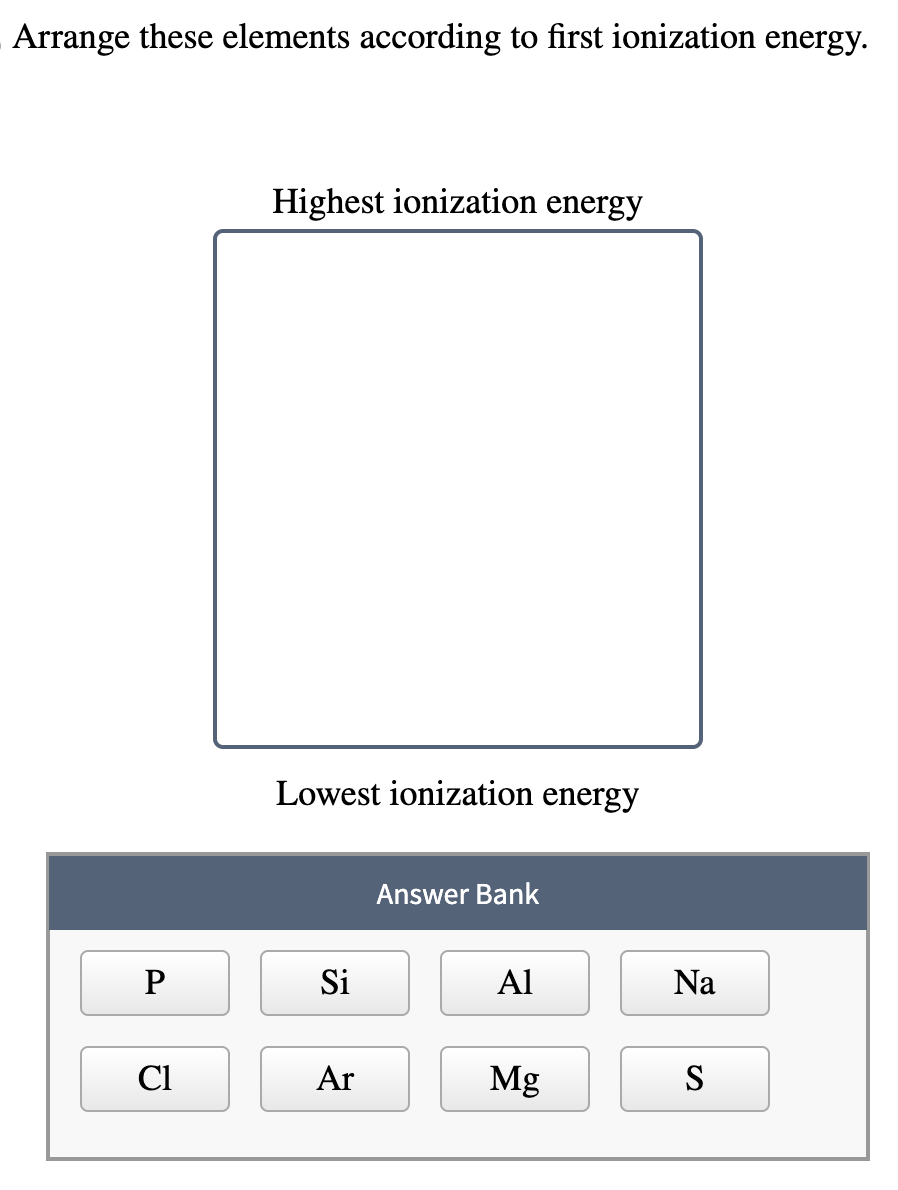
So, the big question: Which of the following has the highest ionization energy? (Drumroll, please!). Let's break it down. Usually, these questions give you a few elements to choose from. To make this fun and educational, let's consider a scenario with four hypothetical elements: Element A, Element B, Element C, and Element D. Ready to play?
Location, Location, Location: The Periodic Table's Secrets
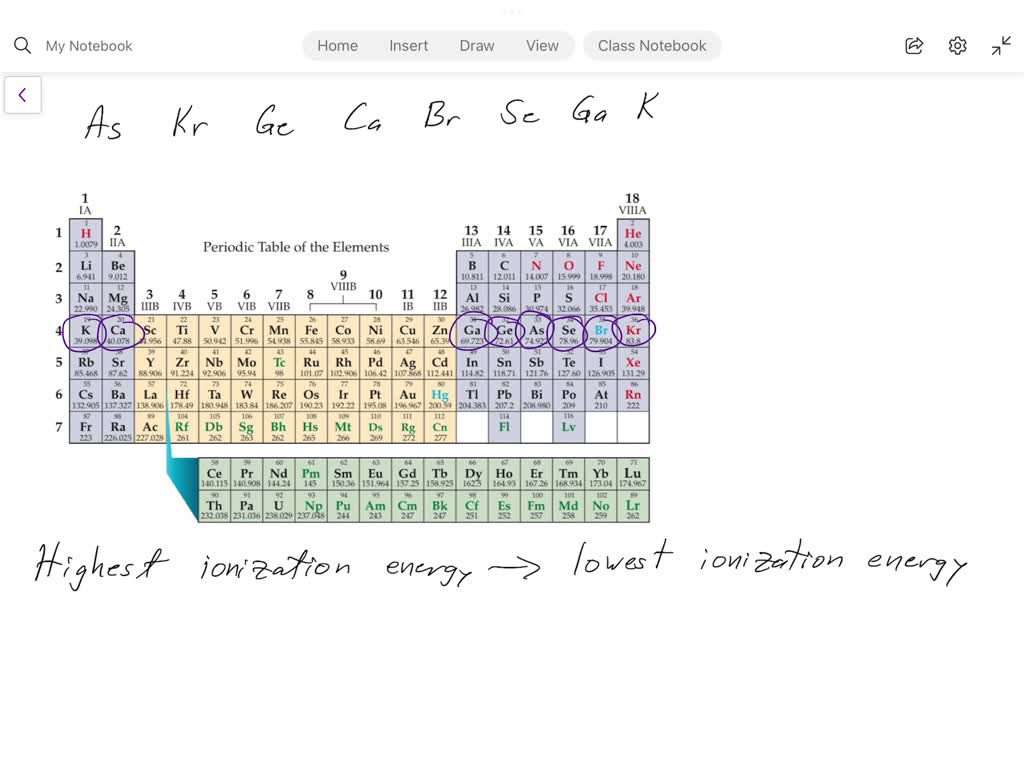
The secret weapon in answering this kind of question is the periodic table! Remember that big chart from chemistry class? It's not just a wall decoration; it's a map to understanding how elements behave. The periodic table basically sorts elements based on their properties and how they interact.
Here’s the key takeaway: Ionization energy generally increases as you move from left to right across a period (a row) and increases as you move from bottom to top in a group (a column). Why? Because of something called effective nuclear charge. Basically, as you move across a period, the nucleus is pulling harder on the electrons, making them tougher to remove.
Think of it like trying to pull a magnet off a fridge. The stronger the magnet, the harder you have to pull! Same idea here.
Shields Up! Atomic Radius and Ionization Energy
Atomic radius is another clue! Elements with a smaller atomic radius (smaller atoms) usually have higher ionization energies. Why? Because the electrons are closer to the positively charged nucleus, held more tightly. Imagine trying to snatch something from someone's hand when they're holding it close to their chest – much harder, right?
Elements with larger atomic radius generally have lower ionization energies. Their electrons are further away from the nucleus, making them easier to pluck away. It’s like reaching for a cookie that’s already sitting on the edge of the table – easy grab!
Full vs. Half-Full: Electron Configuration Matters!
Now, things get a *tiny* bit more complex, but stick with me! Electron configuration, which is how electrons are arranged within the atom, also plays a role. Atoms with completely filled or half-filled electron shells are particularly stable.
Think of it like this: a neatly organized desk is harder to mess up than a completely cluttered one. Similarly, a stable electron configuration makes it harder to remove an electron, resulting in higher ionization energy.
Back to Our Mystery Elements
Okay, so let’s say we know the following about our mystery elements:
- Element A is in Group 1 (Alkali Metals) and Period 3.
- Element B is in Group 17 (Halogens) and Period 3.
- Element C is in Group 2 (Alkaline Earth Metals) and Period 4.
- Element D is in Group 15 (Pnictogens) and Period 2.
Which one has the highest ionization energy?
Based on what we've learned:
- Element B (Halogen) will generally have higher ionization energy than Elements A and C because Halogens are on the right of the periodic table and in the 3rd period.
- Element D (Pnictogen) will have higher ionization energy than Elements A and C because Element D is located to the right and further up the periodic table, which means its electrons are harder to pluck away.
- Comparing Element B and D: Because Element D is further up the periodic table, it would have a greater ionisation energy.
Therefore, the most likely answer is Element D. It's located further up and to the right on the periodic table, which means its electrons are held more tightly!
Why Does This Matter? (Besides Acing Chemistry Tests!)
Okay, so knowing which element has the highest ionization energy might not seem like the most exciting party trick (though, hey, you never know!). But understanding these basic principles opens the door to understanding how the world works at a fundamental level.
It helps us understand how chemical bonds form, why certain reactions happen, and even how materials behave. And that, my friends, is pretty darn cool! It's like unlocking a secret code to the universe.
So go forth, embrace your inner scientist, and keep exploring! The world of chemistry (and all the sciences) is full of fascinating secrets just waiting to be discovered. And who knows, maybe one day you'll be the one making a groundbreaking discovery that changes the world! The journey starts with curiosity, and a willingness to learn. You've got this!