Let's face it, most of us haven't thought about the periodic table since high school chemistry. But stick with me! Understanding where things live on that table – especially the transition metals – is actually surprisingly useful, and even kind of fun, once you see how interwoven they are into our everyday lives. Think of it like knowing the layout of your favorite grocery store. You might not consciously think about it, but knowing where the milk, bread, and snacks are makes shopping so much easier. Similarly, knowing where the transition metals are on the periodic table helps you understand *why* certain materials behave the way they do.
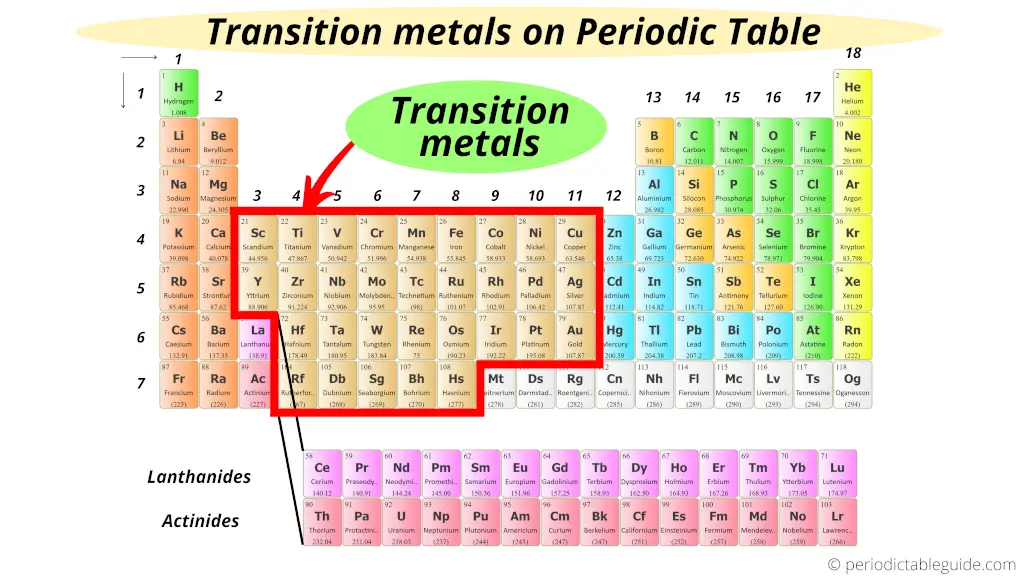
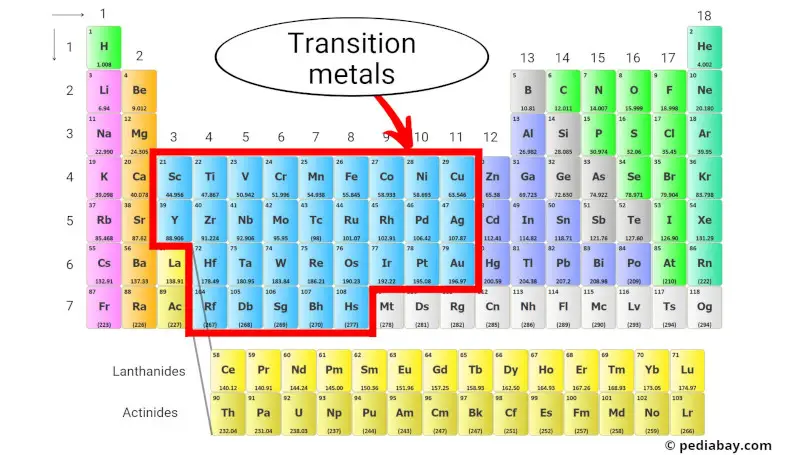
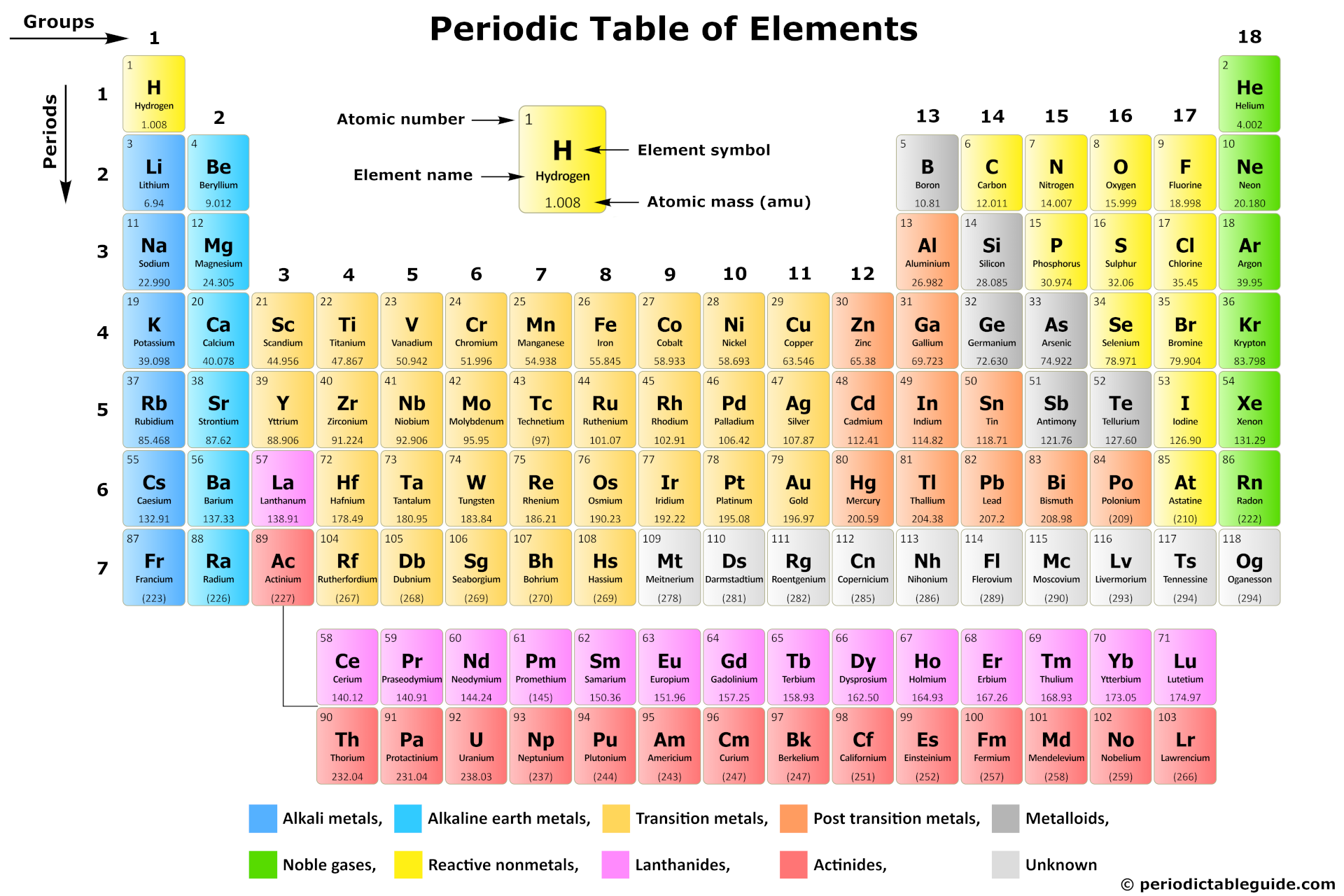
So, where exactly *are* these elusive transition metals? Well, they reside in the **big block in the middle** of the periodic table – specifically, groups 3 through 12. They're sandwiched between the alkali and alkaline earth metals on the left and the main group elements (like oxygen and nitrogen) on the right. Think of them as the *bridge* connecting the reactive metals to the elements that are more crucial for life.
Why should you care? Because transition metals are essential to countless applications! They're the workhorses of modern technology and industrial processes. For example, iron (Fe), a transition metal, is the backbone of our infrastructure. From the steel in our buildings and cars to the nails holding our furniture together, iron is everywhere. Copper (Cu), another transition metal, is the go-to material for electrical wiring because of its excellent conductivity. And let's not forget titanium (Ti), known for its strength and lightness, which makes it ideal for aerospace applications and even medical implants.
Beyond the obvious, transition metals play crucial roles in our very existence. Hemoglobin, the protein in our red blood cells that carries oxygen, contains iron. Enzymes, the catalysts that speed up biochemical reactions in our bodies, often rely on transition metals like zinc (Zn) or manganese (Mn) to function correctly. Even the vibrant colors you see around you are often thanks to transition metal compounds! Think of the deep blue of cobalt glass or the vibrant green of chromium oxide in paints.
So, how can you better appreciate the transition metals? First, visualize the periodic table. Picture that central block and remember that's where the magic happens. Second, start noticing the materials around you. Is your stainless steel cutlery magnetic? That's because of the iron content! Is your jewelry made of gold or silver? Those are transition metals too! Third, when reading about new technologies or scientific discoveries, pay attention to the elements involved. You'll likely find that transition metals are playing a *key role*.
Finally, don't be afraid to dive deeper! There are tons of online resources, videos, and even interactive periodic tables that can help you explore the fascinating world of transition metals. You might be surprised at how much they impact your life, and how much there is to learn about these versatile and essential elements. Exploring the periodic table doesn't need to feel like homework, approach it with curiosity and you'll find a world of fascinating chemistry hiding in plain sight!