Ever looked at a periodic table and thought, "Wow, that's… a lot of boxes"? Yeah, me too. But hidden within that grid of elements lies a real treasure trove – the transition metals. Now, before your eyes glaze over, stick with me! These aren't your average, run-of-the-mill elements. They're the workhorses of the chemical world, and knowing where to find them on the periodic table is like having a secret map to all sorts of cool stuff.
The Periodic Table Neighborhood: Finding Our Way
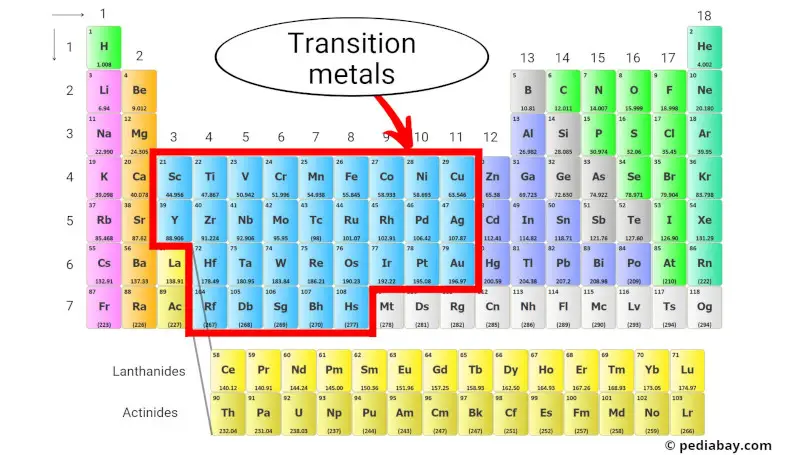
So, where do we find these intriguing metals? Think of the periodic table as a neighborhood. You've got the super-reactive Alkali Metals hanging out on the far left, and the chill Noble Gases all the way on the right. The transition metals? They're smack-dab in the middle, in what's called the d-block. Seriously, right in the thick of it all! Think of them as the bustling downtown area, where all the action happens.
More specifically, you'll find them in groups 3 through 12. Just picture it: scandium (Sc) all the way to zinc (Zn), yttrium (Y) to cadmium (Cd), and so on, all lined up like soldiers ready for action. You can't miss 'em!
Why Are They Called "Transition" Metals Anyway?
Good question! The name “transition” comes from their position on the periodic table. They literally transition between the highly reactive elements on the left and the less reactive ones on the right. It’s like they're the bridge between two worlds. Pretty neat, huh?
But it's more than just their location that earns them the title. Transition metals have some funky electron configurations, meaning their electrons are arranged in ways that give them a wide range of properties. This allows them to form colorful compounds, act as catalysts in chemical reactions, and generally be awesome all-around players in the chemical world.
What Makes Them So Special? Exploring Their Properties
Okay, let's get into the nitty-gritty of why transition metals are so cool. One of the most striking features is their ability to form compounds with vibrant colors. Think of the deep blue of copper sulfate crystals, the fiery orange of potassium dichromate, or the rich green of nickel salts. These colors aren't just pretty to look at; they're a direct result of how the electrons in transition metals interact with light.
And then there's their catalytic activity. Many transition metals and their compounds are excellent catalysts, meaning they can speed up chemical reactions without being consumed in the process. This is crucial in industries ranging from oil refining to pharmaceuticals. Imagine them as the little matchmakers of the chemical world, bringing molecules together to form new and exciting partnerships!
Another remarkable property is their ability to form compounds with multiple oxidation states. What does that mean? Simply put, a single transition metal can form bonds with different numbers of other atoms, leading to a diverse range of compounds with varying properties. This versatility is part of what makes them so useful in a wide variety of applications. It's like having a Swiss Army knife in your toolkit – always ready for whatever the situation demands.
Everyday Heroes: Where You Find Transition Metals
So, where do you encounter these transition metals in your everyday life? More places than you probably realize! Iron (Fe) is in your blood, helping to carry oxygen throughout your body. Titanium (Ti) is in everything from airplanes to artificial joints, thanks to its strength and lightweight properties. Copper (Cu) is in your electrical wiring, conducting electricity with high efficiency. Nickel (Ni) is in stainless steel, giving it its corrosion resistance.
And let's not forget gold (Au), silver (Ag), and platinum (Pt) – the precious metals that are used in jewelry, electronics, and even catalytic converters in cars. These metals are valued not only for their beauty but also for their unique chemical and physical properties. They're the celebrities of the transition metal world, gracing everything from red carpets to cutting-edge technology.
Beyond the Basics: Diving Deeper
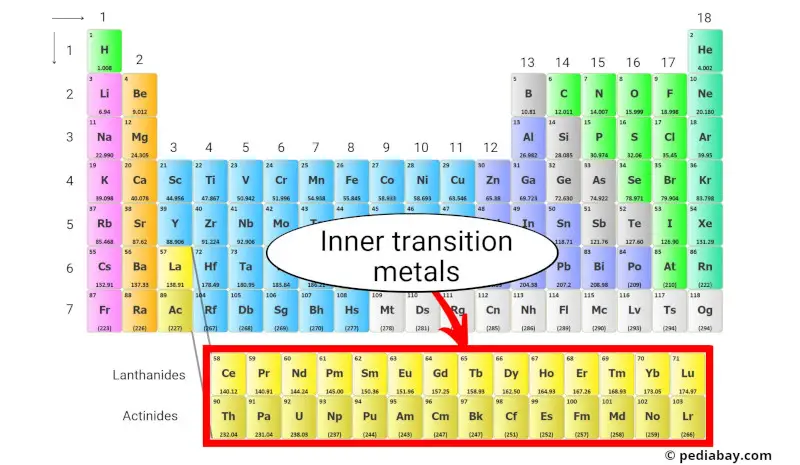
Want to go even deeper into the world of transition metals? Consider delving into the lanthanides and actinides, which are often shown below the main body of the periodic table. These elements, sometimes called inner transition metals, also exhibit similar properties to the transition metals and play important roles in various fields, including nuclear energy and medical imaging.
Exploring the electronic configurations of transition metals can also provide a deeper understanding of their properties. The arrangement of electrons in the d orbitals is responsible for many of their unique characteristics, such as their color and magnetic properties. It's like looking under the hood of a car to see how all the parts work together.
So, next time you glance at the periodic table, don't just see a boring grid of elements. Remember the transition metals, the versatile and colorful elements that play a crucial role in our world. They're the unsung heroes of chemistry, quietly working behind the scenes to make our lives better in countless ways. And now, you know exactly where to find them!