Okay, science enthusiasts and curious minds! Let's talk about something that might sound intimidating but is actually pretty cool: the transition elements. You know, those guys hanging out in the middle of the Periodic Table, looking all mysterious and metal-like?
The Periodic Table Neighborhood: Finding Transition Town
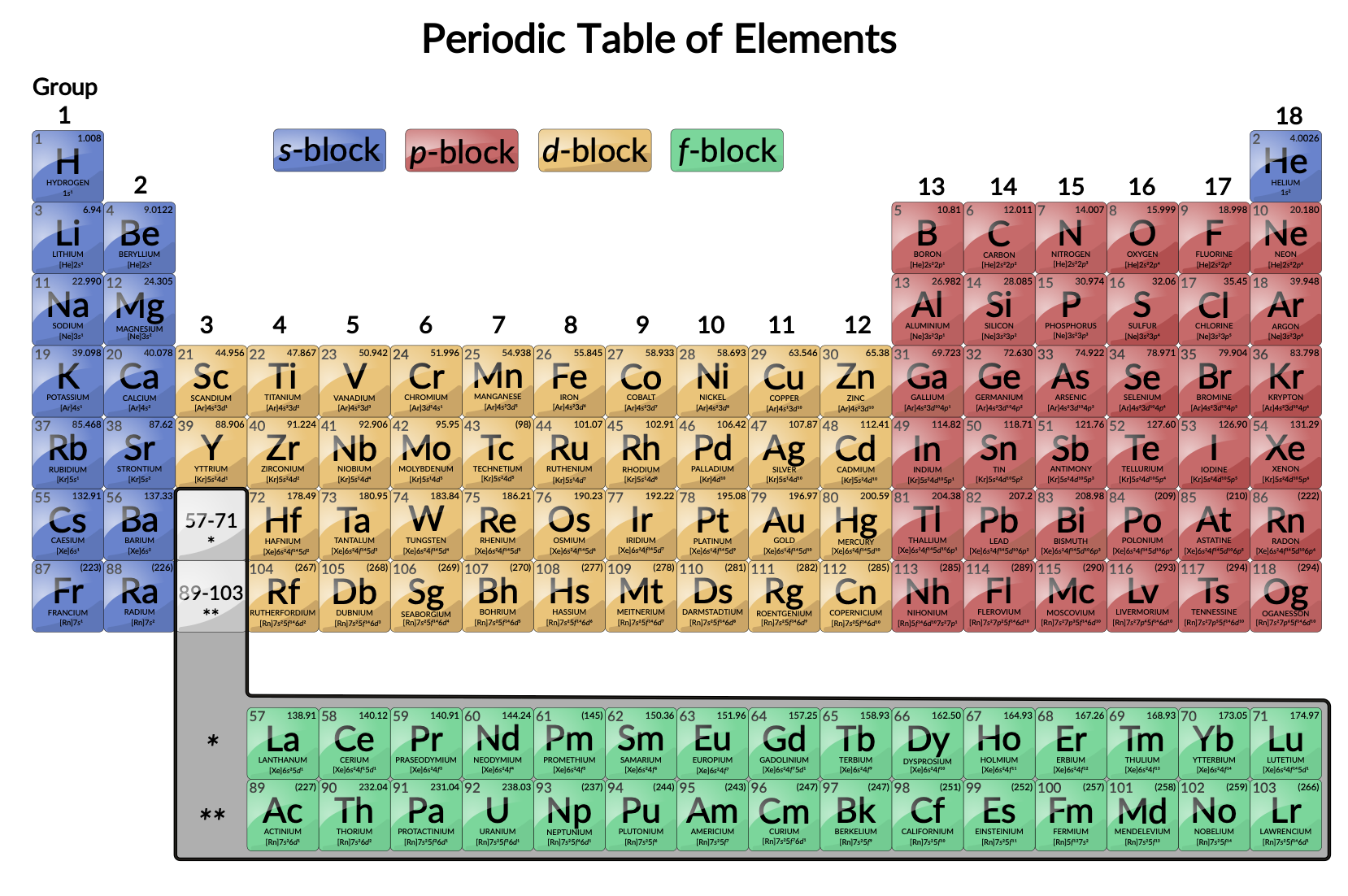
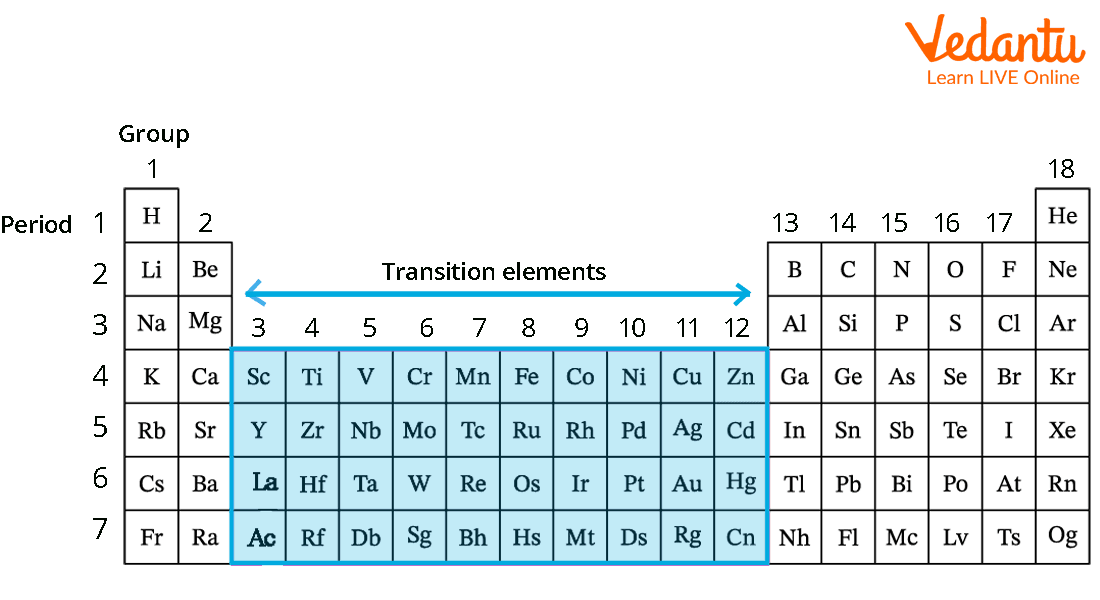
Think of the Periodic Table as a well-organized neighborhood. You've got your super-reactive alkali metals in one cul-de-sac, the noble gases chilling on the other side of the street in their gated community, and then you have the transition metals in the *big* central block. Specifically, they occupy groups 3 through 12.
Imagine the Periodic Table as a city map. The transition elements are downtown, the bustling center of activity, where all the interesting chemistry happens. You can't miss them – they're the large, colorful section that acts as a bridge between the more reactive elements on the left and the less reactive ones on the right.
Why Are They Called "Transition" Elements?
Great question! They're called "transition" because they represent a transition between the strongly electropositive elements (like sodium and potassium) and the electronegative elements (like chlorine and oxygen). They act as a kind of chemical bridge, exhibiting properties that are *in between* those extremes.
What Makes Them So Special? The Chemistry Lowdown
So, what makes these elements so unique? Well, a few things. Firstly, many of them can exist in *multiple oxidation states*. That means they can form different types of chemical bonds and compounds. Think of it like they have many different outfits they can wear, each suited for a different occasion!
Another cool feature? Many transition metal compounds are colorful! Copper compounds are often blue or green, chromium compounds can be vibrant shades of green, yellow, and orange, and manganese compounds can be purple or pink. This is because of the way their electrons absorb and reflect light.
Fun Fact: Remember that ruby necklace from your favorite movie? The red color comes from chromium ions (Cr3+) within the aluminum oxide crystal structure!
Transition Metals in Real Life: More Than Just Lab Coats
You interact with transition elements every single day, often without even realizing it! Here are a few examples:
- Iron (Fe): The backbone of steel, used in everything from buildings and bridges to cars and cutlery. Without iron, we'd be living in a very different world! And, you know, your blood wouldn't be able to carry oxygen. Pretty important stuff.
- Copper (Cu): Used in electrical wiring, plumbing, and even in some antibacterial surfaces. Those shiny copper pots and pans in your kitchen? Transition metal magic at work.
- Titanium (Ti): Strong, lightweight, and corrosion-resistant, titanium is used in aircraft, medical implants, and even in some sporting equipment like golf clubs. It's the superhero of metals!
- Gold (Au) and Silver (Ag): Jewelry, electronics, and even some medications. These precious metals have been valued for centuries for their beauty and unique properties.
- Zinc (Zn): Found in sunscreen, batteries, and as a protective coating on other metals to prevent rust (galvanization). It's the unsung hero protecting our stuff!
Pro Tip: Next time you're cooking, take a look at your pots and pans. Chances are, they contain stainless steel, which is an alloy containing iron, chromium, and nickel – all transition elements!
Beyond the Basics: Delving Deeper
If you're feeling ambitious, you can dive deeper into the electronic configurations and d-orbital splitting of transition metals. But for now, just appreciate their versatility and the crucial role they play in our world.
The *Lanthanides* and *Actinides*, also located towards the bottom of the Periodic Table, are often considered inner transition elements and have fascinating properties of their own worthy of later exploration!
Final Thoughts: Transitioning to Appreciation
So, there you have it: a quick and easy tour of the transition elements on the Periodic Table. From the iron in our blood to the gold in our jewelry, these elements are essential to our lives and play a vital role in countless technologies and industries. Next time you see something made of metal, take a moment to appreciate the amazing chemistry that makes it all possible. It's a reminder that even the seemingly complex world of science is interconnected with our everyday experiences. Embrace the transition!