Okay, so picture this: me, staring blankly at a periodic table that looked less like a helpful guide and more like a cryptic crossword puzzle. I was trying to remember where the heck those super-reactive metals were… you know, the ones that go boom if you even *think* about adding water. It was chemistry exam season. Good times. NOT.
Finally, after what felt like an eternity (and fueled by copious amounts of caffeine), it clicked. And I'm here to make sure you don't suffer the same periodic table-induced trauma. Today, we're talking about the alkali metals! Let’s break down where to find these guys.
The Land of Shiny and Explosive: Group 1
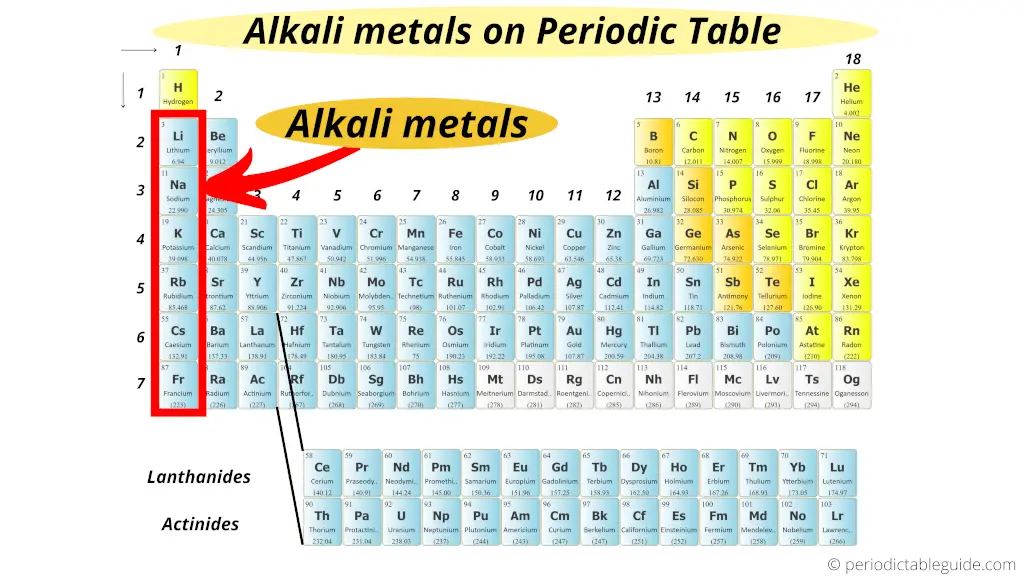
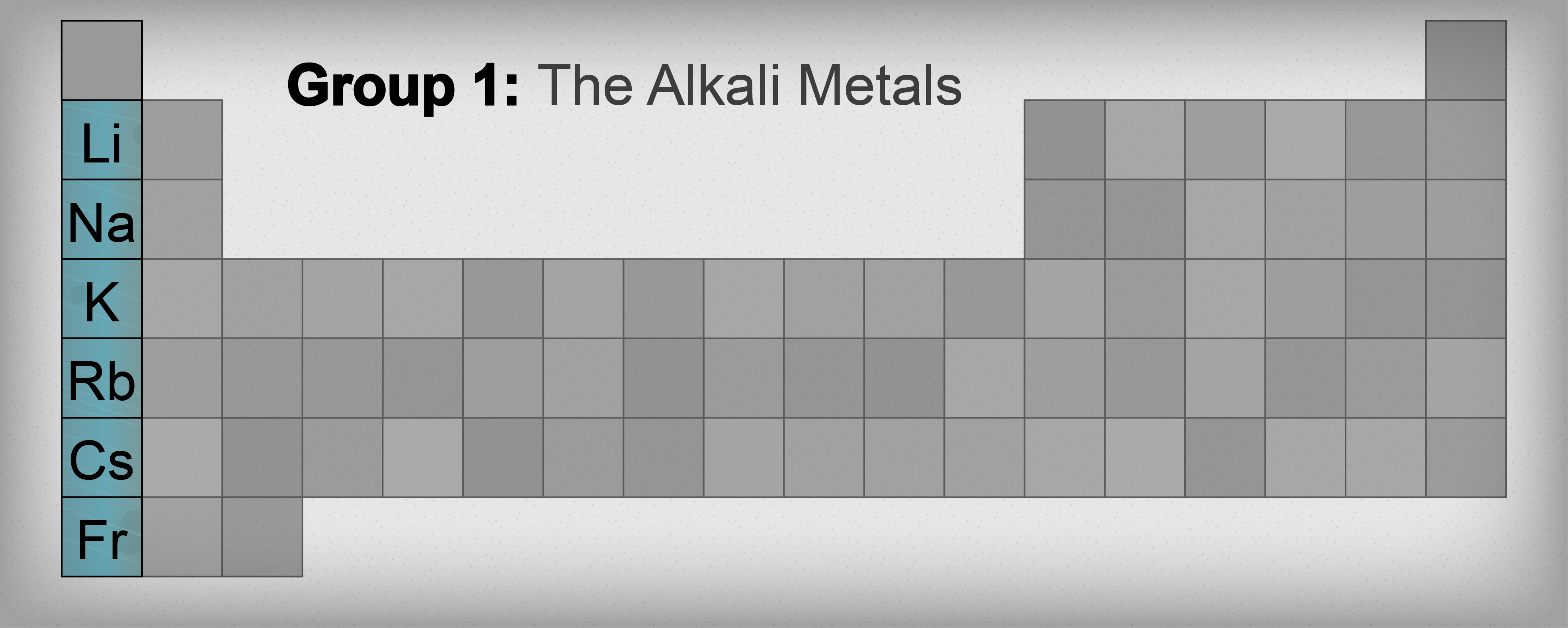
Drumroll, please! The alkali metals live in Group 1 of the periodic table. That's the very first column on the left-hand side. Easy peasy, right? Well, almost. There's a bit of a catch.
See that hydrogen (H) chilling at the top of Group 1? Yeah, it's a bit of a poser. While it *is* in Group 1, it's not actually an alkali metal. It's a nonmetal! Think of it as the awkward guest who showed up to the party but doesn't quite fit in. So, hydrogen's just hanging out there at the top, being hydrogen.
(Side note: Hydrogen is actually super important and unique in its own right, but that's a story for another day. Focus, grasshopper!)
The Alkali Metal Lineup: Who's Who?
Alright, let's meet the actual alkali metal crew. Starting from the *second* row of Group 1, we've got:
- Lithium (Li): Used in batteries and mood stabilizers! Who knew such a reactive element could be so… therapeutic?
- Sodium (Na): Found in table salt (NaCl) and essential for nerve function. Keep this one away from water!
- Potassium (K): Another vital nutrient, also important for nerve and muscle function. Fun fact: it's even more reactive than sodium.
- Rubidium (Rb): Used in atomic clocks. So, if you’re super concerned about being precisely on time, thank rubidium!
- Caesium (Cs): Also used in atomic clocks, even *more* accurate than rubidium ones. Like, ridiculously precise.
- Francium (Fr): This one's a bit of a loner. It's incredibly rare and radioactive. We're talking vanishingly small amounts. You're highly unlikely to ever encounter it in real life.
So there you have it – the alkali metal A-team. Just remember to start from Lithium, skipping Hydrogen.
(Pro tip: create a mnemonic to remember the order! Like "Little Ninjas Kick Red Cats, Frequently!" Okay, maybe not the *best* mnemonic, but you get the idea. Get creative!)
Why Are They So Special?
Good question! Alkali metals are notoriously reactive because they have just *one* valence electron (an electron in their outermost shell). They're desperate to get rid of it to achieve a stable electron configuration (like the noble gases). This eagerness to lose that electron is what makes them so willing to react with, well, just about everything.
That’s why you'll find them in compounds rather than as pure elements in nature. They are always bonded to something else.
And that reactivity? That's why they're stored under oil. Prevents unwanted explosions. Nobody wants that.
(Just a little chemistry humor for ya!)
In Conclusion (and hopefully no more blank stares)
So, there you have it. Alkali metals reside in Group 1 of the periodic table (minus hydrogen, the imposter). They're shiny, reactive, and play important roles in various applications, from batteries to atomic clocks. Next time you see a periodic table, you'll know exactly where to find these elemental superstars. And hopefully, you won't have to suffer through the same chemistry exam-induced panic I did!
Now go forth and conquer that chemistry knowledge!