Ever wonder why your phone charges, your lights turn on, or your toaster toasts? Electricity! And a big part of that story is thanks to amazing metals like copper. But what makes copper so special?
Free Electrons: The Secret Sauce
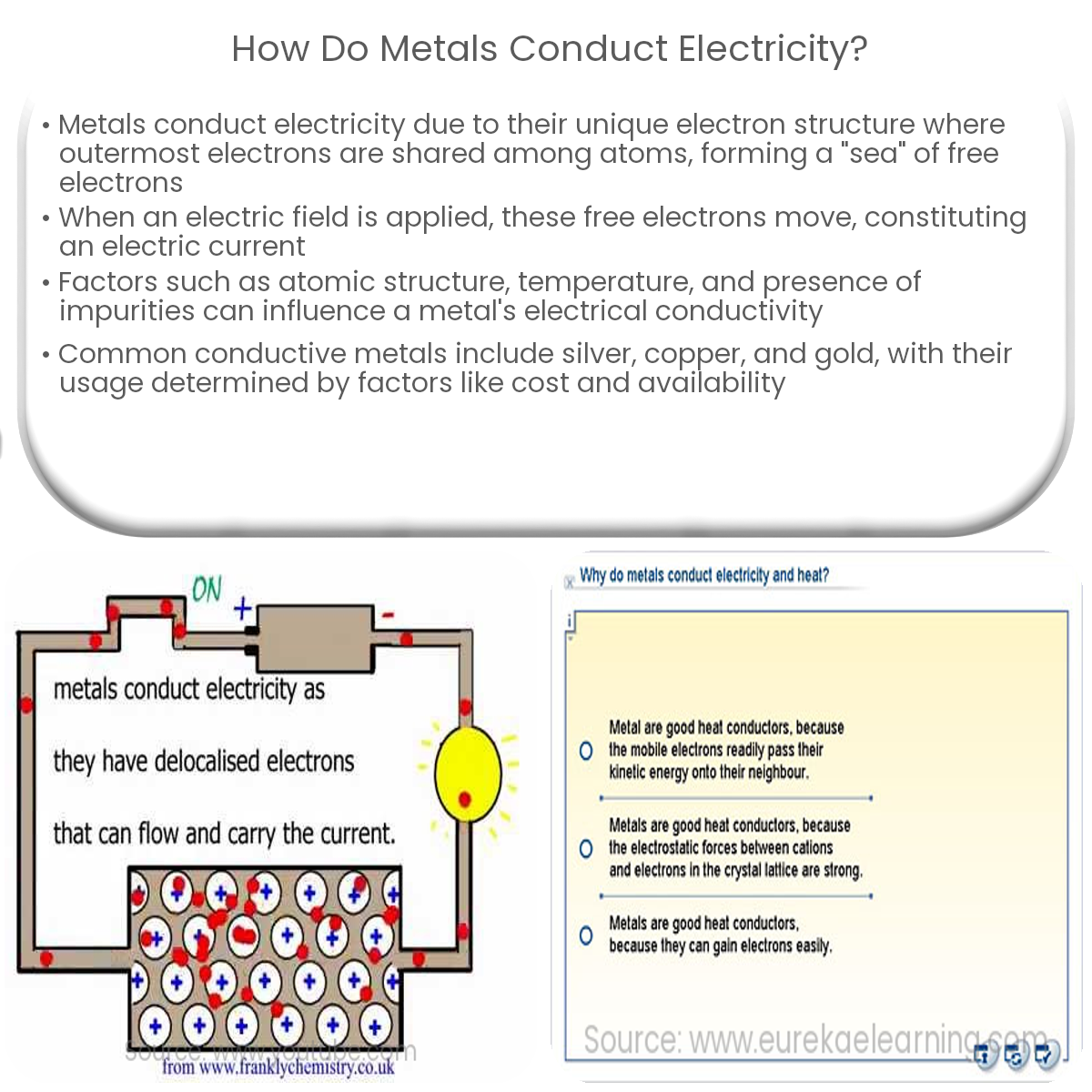
Imagine copper atoms as tiny little cities. Each city has residents called electrons.
Now, here's the cool part. Some of these electrons in copper aren't tied down! They're like free-spirited travelers, wandering the streets.
What Makes Them Free?
It's all about the atom's structure. Copper atoms have a particular arrangement that allows some electrons to roam freely. Think of it like loose luggage on a baggage carousel – ready to be moved!
These "free electrons" aren't really free in the sense that they can escape the copper entirely. But they're not tightly bound to a single atom. They can hop from one copper atom to another with relative ease.
This abundance of mobile electrons is the key to copper's conductivity. It's what makes it a superhighway for electricity!
The Electron Shuffle: How Electricity Flows
So, what happens when you plug something in? You're essentially creating an electrical "pressure" – a voltage.
This pressure acts like a crowd herding those free electrons in copper. They all start moving in the same direction.
It's like a giant electron shuffle! One electron bumps into the next, pushing it along. This chain reaction continues down the wire.
Not a Sprint, But a Drift
You might think electrons are zooming at lightning speed. Actually, they're drifting at a surprisingly slow pace! However, the effect is instantaneous.
Think of it like a pipe filled with water. When you push water in one end, water comes out the other end almost immediately, even though the individual water molecules aren't racing through the pipe.
The same principle applies to electrons in copper. The "push" of voltage causes a near-instantaneous flow of electrical energy.
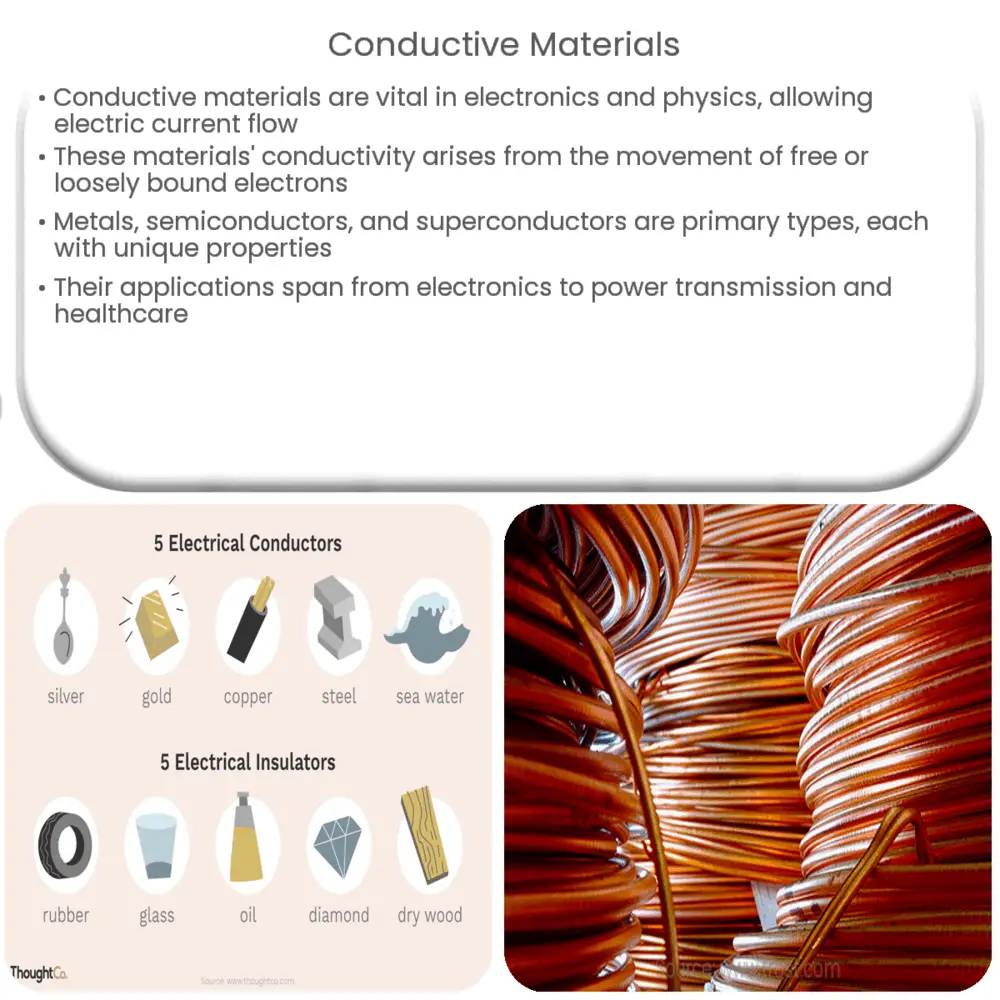
Other Metals and Conductivity
Copper isn't the only conductive metal. Silver is actually an even better conductor, but it's too expensive for everyday wiring.
Gold is another excellent conductor, often used in electronics where corrosion resistance is crucial.
Aluminum is also commonly used, especially for high-voltage power lines. It's lighter and cheaper than copper, though not quite as conductive.
Why Aren't All Metals Conductive?
The difference lies in the atomic structure. Some metals have very tightly bound electrons. These electrons are not free to move and carry charge.
Think of it like a crowded dance floor versus an open field. It's much easier to move around in the open field!
In metals like iron, some electrons are free, but not as freely as in copper. That's why iron is a less efficient conductor.
Copper in Our World: A Conductivity Champion
Copper is everywhere! It's in the wiring of your house, the circuits of your phone, and even in some of your cookware.
Its excellent conductivity makes it ideal for transmitting electricity efficiently. It doesn't lose much energy as heat.
This is important because wasted energy is wasted money and adds to environmental concerns.
More Than Just Wires
Copper is also used in electric motors, generators, and transformers. It plays a crucial role in creating and distributing electricity.
Even the tiny coils inside your smartphone's speakers rely on copper to produce sound.
Copper's versatility and affordability make it an indispensable material in modern technology.
The Joy of Understanding: Seeing the Invisible
Understanding how copper conducts electricity is like unlocking a secret code. It reveals the hidden workings of the world around us.
It allows us to appreciate the ingenuity of engineers and scientists who have harnessed these properties for our benefit.
Next time you flip a light switch, take a moment to think about the amazing electron shuffle happening inside those wires. It's a small miracle of physics in action!
Explore Further!
There are tons of resources online to delve deeper into the world of conductivity. Check out some science websites or even experiment with simple circuits (under safe supervision, of course!).
You might be surprised at how fascinating and accessible this topic can be.
Understanding the science behind everyday phenomena can be incredibly rewarding. So go ahead, explore the wonderful world of conductivity!
Beyond Conductivity: Other Cool Copper Properties
Copper isn't just a conductivity superstar! It has other awesome traits too.
It's highly ductile, meaning it can be easily drawn into wires. Try doing that with a rock! Good luck!
It's also malleable, which means it can be hammered into thin sheets. Think of copper roofing.
Corrosion Resistance: A Long-Lasting Metal
Copper is relatively resistant to corrosion. This means it doesn't rust easily like iron. That's why you see copper pipes used in plumbing.
The Statue of Liberty is a prime example! Its outer layer is made of copper. Over time, it has developed a greenish patina, which actually protects the underlying metal.
This durability makes copper a valuable and long-lasting material.
Copper and the Environment: A Sustainable Choice
Copper is also recyclable. This is a huge plus for the environment!
Recycled copper retains its excellent properties. It can be reused in countless applications.
Recycling copper reduces the need for mining new ore, which can have significant environmental impacts.
A Circular Economy Star
Copper fits perfectly into the concept of a circular economy. Materials are reused and repurposed to minimize waste.
This makes copper a sustainable choice for a variety of industries.
So, when you choose products made with copper, you're also supporting a more environmentally responsible approach.
So, What Have We Learned?
Copper's amazing conductivity boils down to its free electrons. These electrons can easily move and carry electrical charge.
This makes copper a crucial component of countless technologies. From power grids to smartphones, we rely on its unique properties.
It is also easy to recycle, making it environment friendly.
A World of Wonder
Understanding the science behind everyday materials like copper opens up a world of wonder.
It encourages us to appreciate the ingenuity of human innovation and the beauty of the natural world.
Hopefully, this article has sparked your curiosity and inspired you to explore even more about the fascinating world of science and technology!