Alright, settle in folks, grab your lattes, because we're about to tackle something that sounds way more complicated than it actually is: Polymers! I know, I know, the word itself sounds like something a supervillain would yell before unleashing their latest doomsday device. But trust me, it’s way cooler (and less destructive) than that.
Imagine a bunch of LEGO bricks. Not just a sad, lonely few, but like… the entire inventory of a LEGO warehouse. Now, imagine you're building something awesome – maybe a replica of the Eiffel Tower, or a life-sized dragon (ambitious, I know!). Each individual LEGO brick is small and relatively simple, right? But when you link them all together in a specific way, you get something much bigger and way more impressive. That, my friends, is essentially what a polymer is!
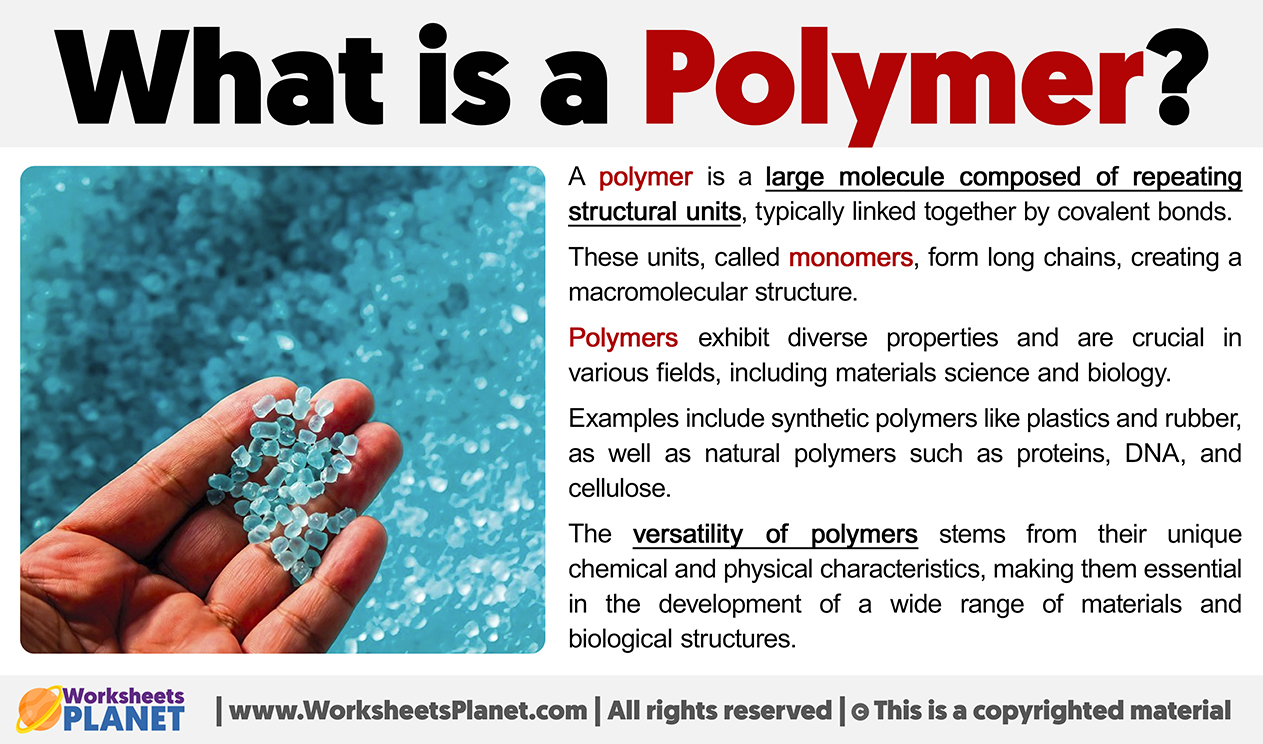
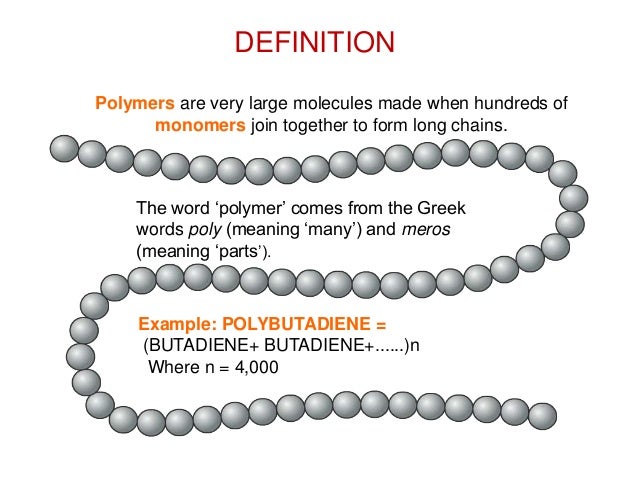
So, what’s the super-scientific definition? (Brace yourselves, it's not THAT scary). A polymer is a large molecule – a macromolecule, if you want to impress your friends at your next trivia night – made up of repeating smaller units called monomers. Think of the monomers as those individual LEGO bricks, and the polymer as the awesome LEGO creation.
Basically, it’s a bunch of identical (or sometimes slightly different) small molecules chained together. And how long are these chains? Some are short and sweet, others are so ridiculously long they make the Great Wall of China look like a garden fence! These chain lengths have a massive impact on the polymer’s properties – making it strong, flexible, stretchy, or even gooey (more on that later).
Natural vs. Synthetic: The Polymer Showdown!
Now, polymers aren’t some newfangled invention cooked up in a lab by mad scientists (although, some *are* cooked up in labs by very dedicated scientists). Polymers are everywhere! Mother Nature has been rocking the polymer game for billions of years.
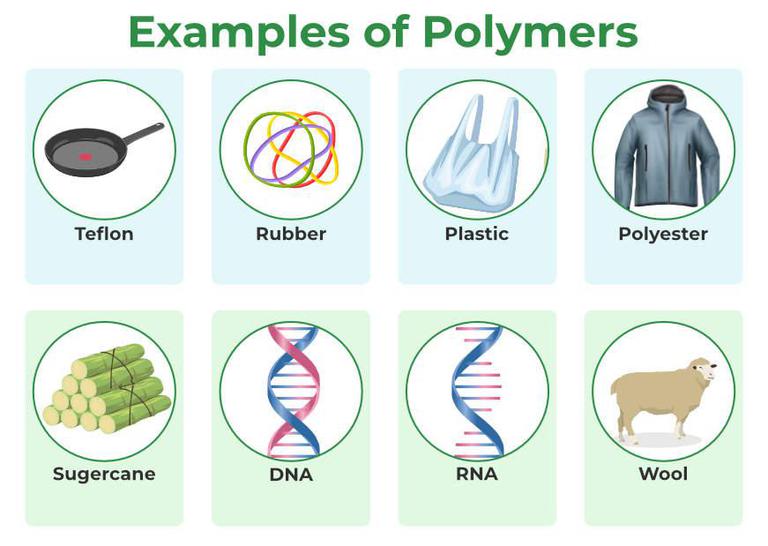
Think of cellulose, the stuff that makes up the walls of plant cells. That's a natural polymer! Wood, cotton, paper – all thanks to cellulose. And what about starch? That's the energy storage polymer in plants, which means that delicious potato you're planning for dinner is essentially a polymer powerhouse. Even your own DNA is a polymer, carrying all the genetic information that makes you… well, you!
But then there are the synthetic polymers – the ones created by humans. These are the plastics, the rubbers, the fibers that make up so much of our modern world. Polyethylene (that's the stuff in plastic bags), nylon (think stockings and parachutes), and polyester (hello, comfy clothing!) – all synthetic polymers. Some scientists even joke that we are now living in the "Plasticene" geological epoch due to the sheer volume of plastic (a polymer) we've produced!
Properties and Uses: More Than Just Plastic Bags!
Okay, so polymers are chains of repeating units. Big deal, right? Wrong! The amazing thing about polymers is that by tweaking the type of monomer used, the length of the chain, and how the chains are arranged, you can create materials with a crazy range of properties. This is why polymers are used in EVERYTHING.
Need something strong and lightweight? Use a polymer like carbon fiber to build an airplane. Need something stretchy and flexible? Rubber, another polymer, is perfect for tires. Want something waterproof and durable? Plastic, ahem, *another* polymer, makes a great raincoat. From medical implants to space suits, polymers are the unsung heroes of modern technology.
And it's not just about the big, industrial applications. Think about the gooey slime you played with as a kid. That was probably a polymer! (Probably involving some sort of questionable borax solution… don’t eat it!). Or the silly putty that could copy newsprint? Yep, another polymer! Polymers are fun, polymers are practical, and polymers are probably in your pocket right now (that smartphone case is likely made of… you guessed it… polymer!).
Here's a fun fact: Spider silk, renowned for its incredible strength and elasticity, is a protein, and proteins are… you guessed it, polymers! Spider-Man’s suit, if it were *actually* made of real spider silk, would be the ultimate in polymer technology (and probably ridiculously expensive).
The Future of Polymers: Beyond Plastic!
Now, I know what you're thinking: “But plastic is bad! It’s polluting the oceans and strangling sea turtles!” And you’re right, the current reliance on some synthetic, non-biodegradable polymers is a serious problem. But the world of polymer science isn't just sitting around twirling its metaphorical mustache while the planet drowns in plastic. Scientists are working hard to develop new, more sustainable polymers.
Think bioplastics made from renewable resources like corn starch or sugarcane. Think polymers that can be easily recycled or even broken down by bacteria. The future of polymers is all about creating materials that are not only useful and versatile but also environmentally friendly. We might even see self-healing polymers that can repair themselves when damaged! Imagine a phone case that fixes its own cracks. The possibilities are endless!
So, next time you hear the word "polymer," don't run screaming for the hills. Instead, take a moment to appreciate the amazing versatility of these giant molecules. They’re the building blocks of everything from trees to toys, and they’re playing a crucial role in shaping the future. Now, if you’ll excuse me, I'm going to go build a LEGO dragon. You know… for science!