Okay, so picture this: I'm at a flea market last weekend, right? And I see this vendor selling what he calls "antique metals." But half of them were clearly painted plastic! I almost lost it. It got me thinking, like, what actually makes something a metal? It's not just being shiny and old, that's for sure. So, let’s dive into the electrifying world of metals, shall we?
What Makes a Metal, Metal? (It's More Than Just Shine!)
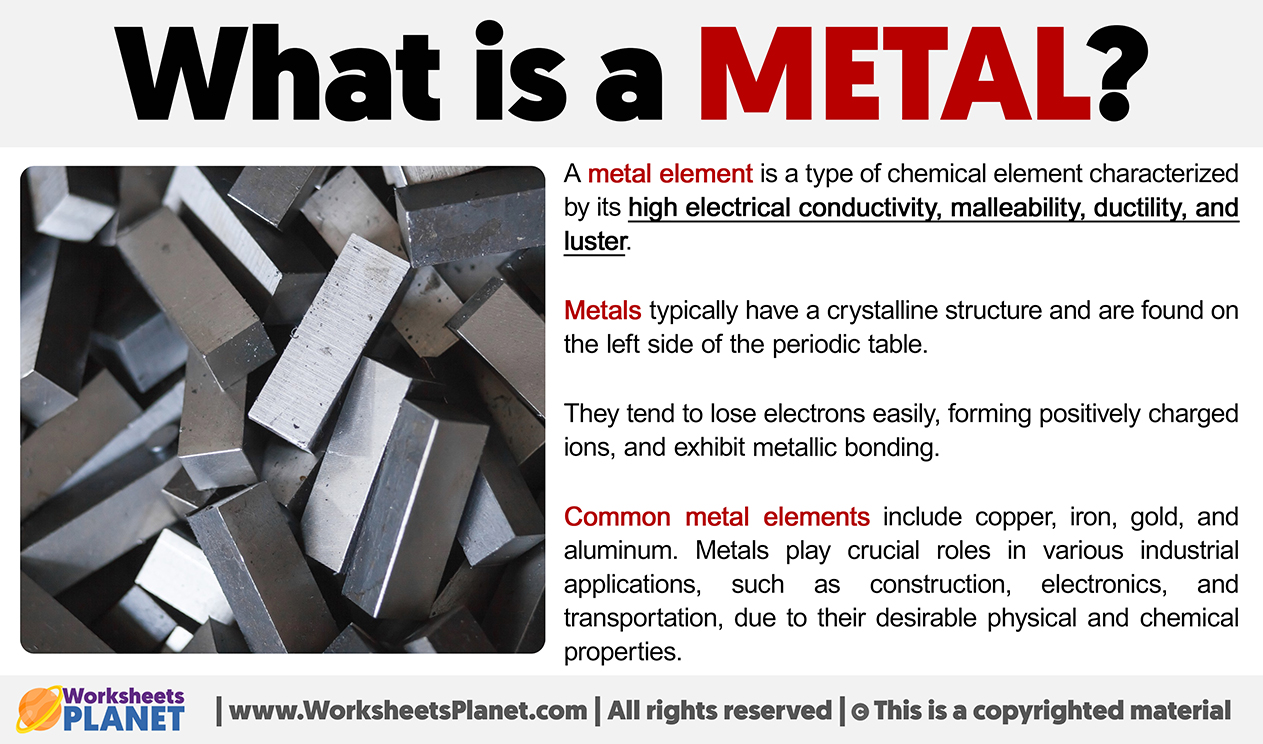
The official, super-sciencey definition? A metal is a substance with high electrical conductivity, high thermal conductivity, and high malleability and ductility. Basically, they're really good at conducting electricity and heat, and you can bash them into shapes without them shattering into a million pieces. Think of copper wiring or a blacksmith hammering out a sword.
But here’s the thing: that’s the technical stuff. Let’s break it down into easier-to-digest chunks.
Conductivity is Key: Ever notice how metal spoons get hot super fast when you stir hot soup? That's thermal conductivity in action. And the fact that electrical wires are made of metal? Electrical conductivity, baby! These qualities are due to the arrangement of electrons in a metal’s atomic structure, allowing them to move freely and easily carry energy.
Side note: Think of electrons as tiny commuters whizzing around a metropolis. Metals are like a well-organized highway system, while other materials are…well, maybe a dirt road riddled with potholes.
Malleable and Ductile: Malleability means you can hammer it into thin sheets (like aluminum foil). Ductility means you can draw it into wires (like, uh, electrical wires…again!). Imagine trying to do that with a rock. *Spoiler alert: it won’t work*. Metals have a unique atomic structure that allows them to deform under pressure without breaking apart. This is because of the way the atoms bond – it's a flexible network rather than a rigid structure.
The Shiny Factor (It's a Perk, Not a Requirement): Okay, most metals *are* shiny, or at least they can be polished to a shine. Think gold, silver, chrome – bling, bling! This is because of how they interact with light. However, some metals tarnish easily (looking at you, silver!), and others might have duller finishes naturally. So, while shininess is a common trait, it's not the ultimate defining characteristic. Don't let a dull-looking material fool you; it could still be a metal!
Beyond the Basics: Metallic Bonding
Want to get a little deeper? Let's talk about metallic bonding. This is the type of chemical bond that holds metal atoms together. It's often described as a "sea of electrons." Each metal atom contributes its outer electrons to this "sea," which are then free to move throughout the entire structure. This electron mobility is what gives metals their excellent conductivity.
Think of it like a mosh pit where everyone is sharing electrons (and maybe a little sweat). It’s a wild party where energy and charge can move freely.
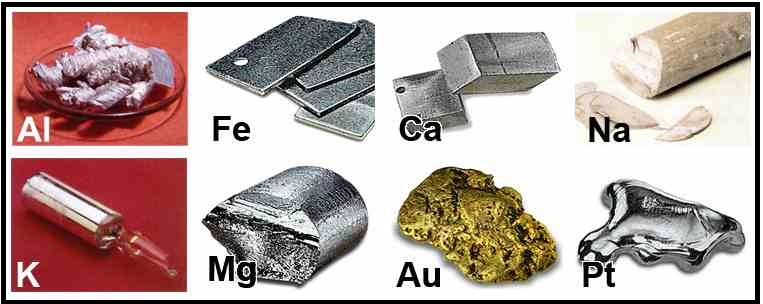
Not All Metals Are Created Equal: Some metals are better conductors than others. Gold and copper are excellent conductors, which is why they’re used in electronics. Steel, on the other hand, is a decent conductor, but it's stronger and more durable, making it ideal for construction. And some metals, like tungsten, have incredibly high melting points, making them perfect for light bulb filaments.
So, is it metal? A Quick Checklist:
Okay, so you find something and you're asking yourself: "Is this a metal?" Here's a quick and dirty test:
- Conductivity Check: Does it conduct electricity or heat well? (Don't go sticking it in a socket, though!)
- Malleability/Ductility: Can you bend it or hammer it without it shattering?
- The Look: Is it shiny, or can it be polished to a shine?
If it ticks most of those boxes, chances are good it’s metal. If it shatters into a million pieces when you try to bend it, or it's a terrible conductor of electricity, you're probably looking at something else entirely. (Probably plastic, if my flea market experience is anything to go by!)
Ultimately, defining "metal" is a bit like defining "art." There are core characteristics, but there's also room for interpretation and exceptions. But hopefully, this gives you a solid foundation for understanding what makes a metal…well, a metal! Now you're ready to go out there and impress all your friends with your newfound knowledge of materials science. You're welcome!