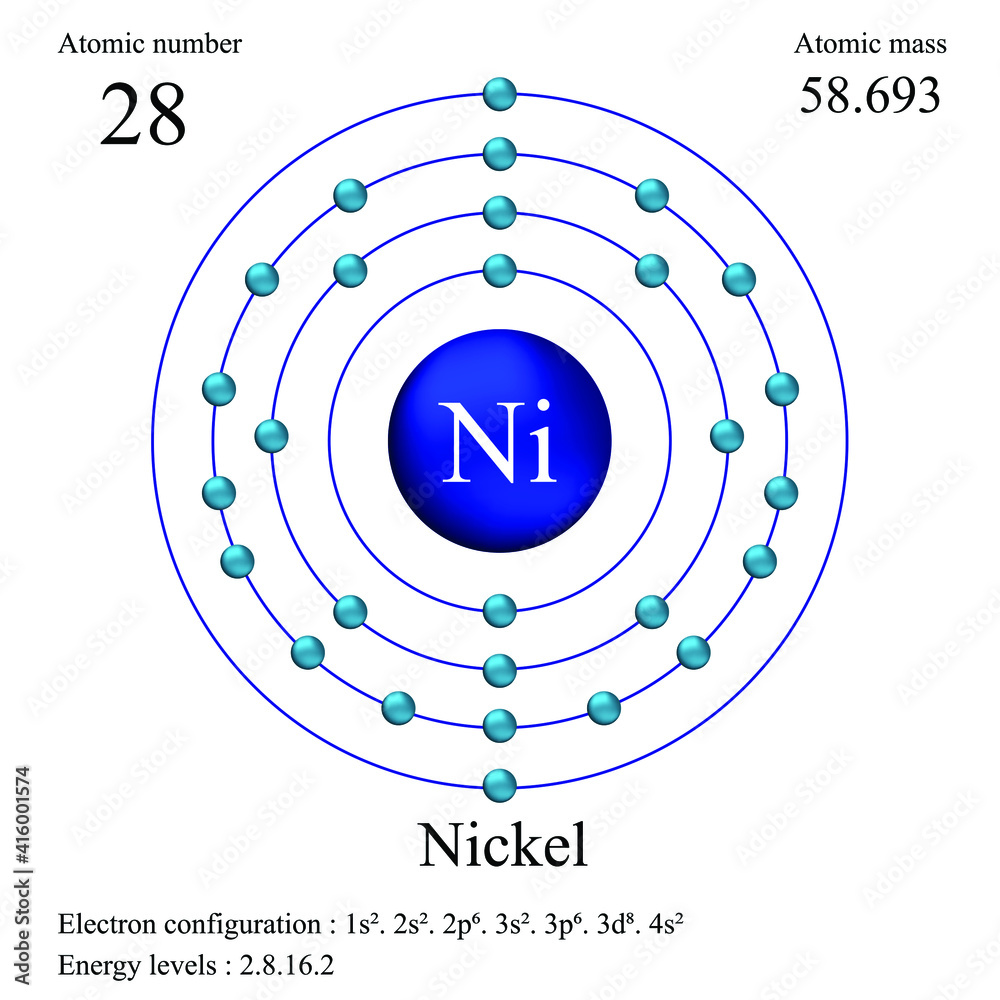
Alright, gather 'round, gather 'round! Let's talk about nickel. Not the kind you find wedged between the couch cushions (though those are valuable in their own way!), but the actual element, hanging out on the periodic table all cool and collected.
Today's burning question, the one that keeps scientists up at night (probably after too much caffeine and late-night experiments gone slightly…explosive): what's the atomic mass of nickel? Buckle up, because we're about to dive into the wonderfully weird world of atoms!
So, What's This "Atomic Mass" Thing, Anyway?
Imagine you're at a cosmic fruit stand. You've got apples, oranges, and… oh, let's say "neutron-berries." The atomic mass is basically the average weight of all the different versions of nickel fruits you can find, taking into account how often you find each version.
Now, in the real world, we're not dealing with fruit, we're dealing with atoms. And instead of apples and oranges, we have *isotopes*.
Isotopes are like slightly different versions of nickel. They all have the same number of protons (which defines them as nickel in the first place – protons are like the official membership card to the "Nickel Club"). But they can have different numbers of neutrons (the "neutron-berries," remember?).
These extra neutrons change the atom's weight, making some nickel atoms a bit heavier than others. It’s like having one apple with a few extra seeds inside. Still an apple, just a bit more… dense.
The Big Reveal: Drumroll Please…
Okay, enough suspense. The atomic mass of nickel is approximately 58.6934 atomic mass units (amu). I know, it's not a round number like 60 or 59. But that's because it's an average, accounting for all those pesky isotopes.
Think of it like this: you surveyed all the people named "Nick" in your town to find their average height. Some are tall, some are short, and the average is probably some weird decimal number. Same deal with nickel!
If someone asks you, just confidently say "About 58.7 amu!" You'll sound like a genius. And you will be, because you read this article.
Why Does Atomic Mass Matter? Is It Going to Help Me Win Jeopardy?
Good question! (And yes, it might help you win Jeopardy if the category is "Chemistry Trivia").
Atomic mass is super important in chemistry for a few reasons:
- Calculating molar mass: If you want to know how much a certain number of nickel atoms weigh (in grams, which is useful for, say, building a giant nickel statue), you need the atomic mass.
- Balancing chemical equations: Making sure you have the right number of atoms on both sides of a chemical equation is crucial for... well, not blowing things up in the lab. Trust me, nobody wants that.
- Identifying elements: Atomic mass, along with the number of protons, helps uniquely identify each element on the periodic table. It’s like a chemical fingerprint.
So, yeah, it's not just a random number. It's a fundamental property that scientists use all the time to understand how the universe works. No big deal.
Nickel's Got Game: Fun Facts About This Shiny Element
While we're on the topic of nickel, let's throw in some amazing (and possibly slightly embellished) facts:
Nickel is magnetic! But not as magnetic as iron. It's more like "attracted-to-magnets-ish." Like a shy puppy who wants to play but isn't quite sure.
Nickel is used in coins! I know, shocking, right? Who would have guessed? But fun fact, the US nickel isn't pure nickel. It's mostly copper. Think of it as nickel in disguise.
Nickel is super resistant to corrosion! That's why it's often used to coat other metals and make them shiny and protected from rust. Nickel is basically the bodyguard of the metal world.
Nickel can be found in meteorites! Yep, space rocks! Some meteorites are made of iron and nickel. So, technically, you could say that nickel is out of this world.
Conclusion: You Are Now a Nickel Expert (Probably)
So, there you have it! You now know the atomic mass of nickel, why it's important, and some fun facts to impress your friends (or at least bore them into submission). Go forth and spread your newfound knowledge of the awesome element that is nickel! Or, you know, just go make a sandwich. Either way, you're awesome!
Just remember, it's all about those protons, neutrons, and averages. And maybe a little bit about space rocks and shiny coins. Now go impress someone with your chemical knowledge!