Okay, so picture this: I'm at a café, right? Ordering my usual ridiculously large iced latte. My friend, bless her heart, is struggling with a stubborn sugar packet. She's wrestling with it, muttering about how everything these days is designed to annoy you. And then, out of the blue, she asks me, "So, what's, like, the *thing* about metals? You know, besides being shiny and occasionally stabbing you in the foot when you stub your toe on a rogue piece of industrial art."
Great question! So, I launch into my explanation, which, let's be honest, probably sounded a little like a caffeinated science teacher who’s had one too many espressos. But I think I got the gist across. And now, for your entertainment, I'm going to do the same for you!
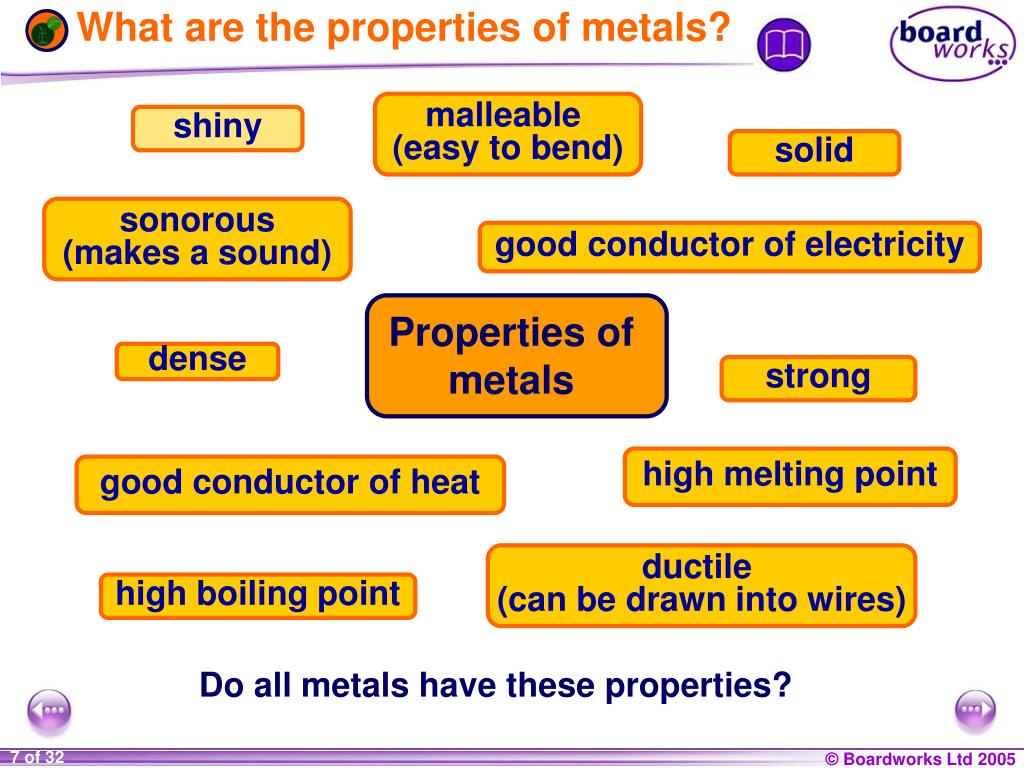
What’s that one thing? Well, if I had to pick the absolute *most* common property of most metals (prepare for a mild science bomb drop), it's their ability to conduct electricity. BAM! Take that, stubborn sugar packet! (I may have been projecting).
Think about it. Why are electrical wires made of copper or aluminum? Because they're metals, and metals are, generally speaking, fantastic at letting electrons zoom through them like tiny, caffeinated race cars. We're talking about electron Grand Prix levels of conductivity!
Why are Metals Such Good Conductors?
Alright, here comes a little science-y explanation, but I promise I’ll keep it entertaining. It all boils down to something called "delocalized electrons." Imagine a bunch of electrons, not tied down to any specific atom, but rather roaming freely through the metal’s structure like tiny, energetic nomads. They're basically having a perpetual electronic party inside the metal.
These roaming electrons are like the ultimate delivery service. When you apply an electrical voltage (think of it as shouting "GO!"), they all start moving in the same direction, carrying the electrical charge along with them. It's like the world’s most efficient – and invisible – postal system. Except, instead of packages, they're delivering electricity. And instead of grumpy postal workers, they're… well, still grumpy electrons probably, but they get the job done!
Important Note: Not *all* metals are created equal in the conductivity department. Some metals are like Olympic sprinters, while others are more like… me trying to run a 5k after eating a whole pizza. Silver is the absolute champion when it comes to conducting electricity, but it’s also ridiculously expensive. That's why you don't see your power lines made of solid silver (unless you're living in a parallel universe where everyone is ridiculously rich and wiring their houses with pure silver is the norm). Copper is a great and more affordable second place.
But Wait, There’s More! (Because Metals are Cool Like That)
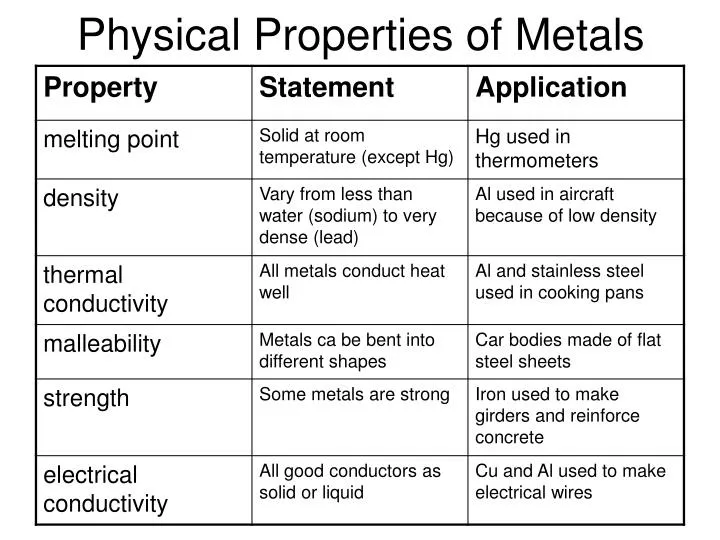
While electrical conductivity is the reigning champion, metals also tend to share a bunch of other interesting properties. Think of them as metal’s supporting cast of characteristics:
- Thermal Conductivity: Metals are usually good at conducting heat too. Ever wondered why your metal spoon gets hot when you leave it in your soup? Blame the electrons! They're not just carrying electricity; they're hauling heat around as well.
- Malleability: This fancy word means that metals can be hammered into thin sheets without breaking. Ever seen aluminum foil? Yeah, that’s malleability in action. Try doing that with a ceramic plate. It'll just shatter. Trust me, I learned that the hard way (don't ask).
- Ductility: This means metals can be drawn into wires. See those electrical wires we were talking about earlier? They're not grown on trees, folks. They're stretched and pulled from metal.
- Luster: Let’s be real, most metals are shiny! This is due to their ability to reflect light. It’s why pirates were always so obsessed with gold. Shiny!
So, there you have it! Metals: electrically conductive, thermally conductive, malleable, ductile, and generally shiny. They’re the unsung heroes of our modern world, powering our homes, holding our bridges together, and even helping us send cat videos across the internet.
Now, if you’ll excuse me, I need to go invent a self-stirring coffee spoon made of solid gold. For… science! Or maybe just because I like shiny things. You decide.
And next time your friend asks you a tricky science question, just remember this story! You'll sound like a genius, even if you're just rambling about caffeinated electrons. Just be sure to order a ridiculously large latte first. For inspiration, of course.