Ever wonder why your phone feels cool to the touch, or why your grandma's cast iron skillet heats up so evenly? It all boils down to the fascinating world of materials, specifically metals, nonmetals, and those sneaky in-betweeners, the metalloids. We interact with these elements and their properties every single day, often without even realizing it.
Think about it: metals conduct electricity, powering our homes and devices. Nonmetals, like the oxygen we breathe, are essential for life. And metalloids? They're the unsung heroes of modern electronics, the key ingredients in your computer chips and solar panels. Understanding their fundamental properties unlocks a deeper appreciation for the world around us.
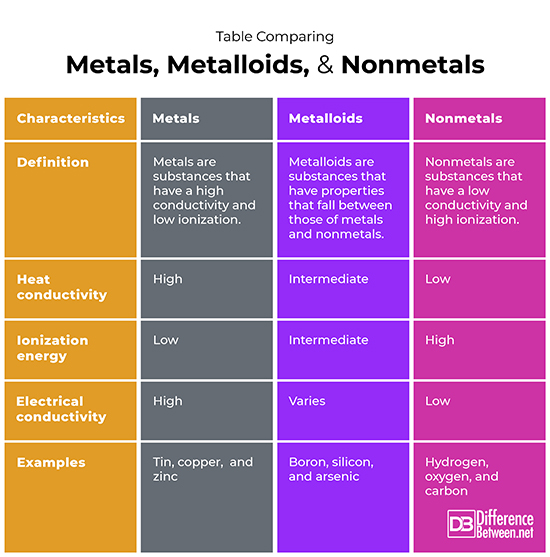
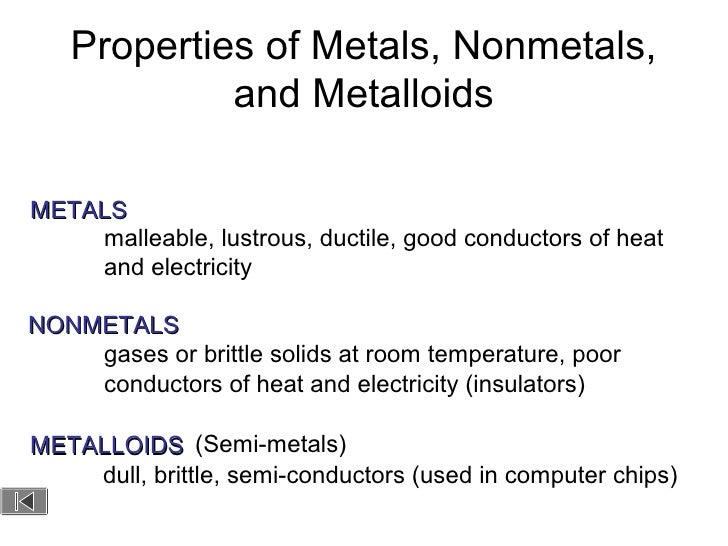
So, what exactly are these properties? Let's break it down. Metals are typically shiny (think gold and silver), malleable (easily shaped, like hammering copper), ductile (can be drawn into wires), and excellent conductors of heat and electricity. Iron, aluminum, and copper are common examples found in everything from buildings to cookware. Their strong metallic bonds give them these remarkable characteristics.
On the other hand, nonmetals are often dull in appearance, brittle (easily broken), and poor conductors of heat and electricity. Think of sulfur, phosphorus, and chlorine. Carbon, in its diamond form, is a notable exception with its extreme hardness, but it remains a poor electrical conductor. Many nonmetals are gases at room temperature, like oxygen and nitrogen, which are crucial components of the air we breathe. Their bonding differs drastically from metals, leading to their contrasting properties.
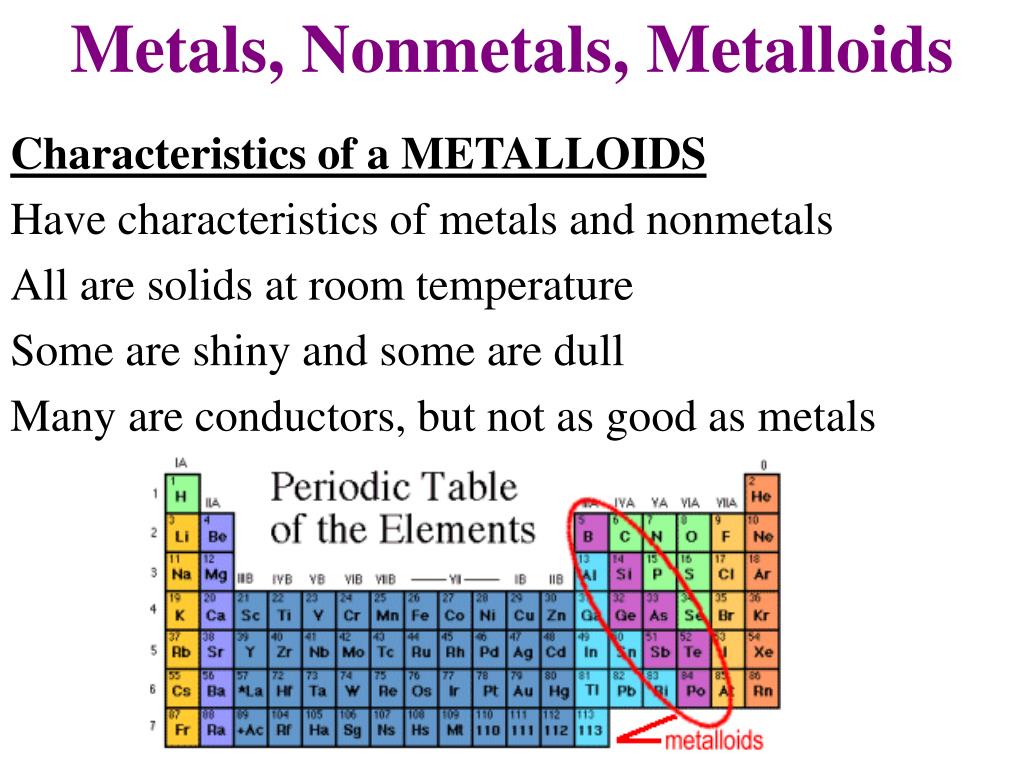
Now for the metalloids, also known as semimetals. They’re the tricksters of the periodic table, possessing properties that fall somewhere between metals and nonmetals. Silicon and germanium are prime examples, exhibiting semiconductivity. This means they can conduct electricity under certain conditions, making them ideal for semiconductors used in transistors and integrated circuits. Metalloids are the foundation of modern electronics, making them incredibly important even if you don't hear about them as often.
How can you better appreciate and understand these elements and their properties in your everyday life? Start by observing. Notice how different materials react to heat. Which pans heat up the fastest? Which materials feel cold to the touch? Try experimenting (safely!) with small circuits and different types of wires to see how conductivity varies. Understanding the building blocks of everything around us, from the simplest spoon to the most complex computer, is a rewarding and enriching experience. The next time you marvel at a sleek, modern gadget, remember the vital role of metals, nonmetals, and metalloids working together in harmony.


:max_bytes(150000):strip_icc()/Metals-Worksheet-56a12db33df78cf772682c48.png)