Ever heard of elements that are so friendly, so eager to mingle, that they'll practically explode with excitement just to hold hands with another atom? Well, buckle up, buttercup, because we're diving headfirst into the wild world of the alkali metals!
The Super Socialites of the Periodic Table
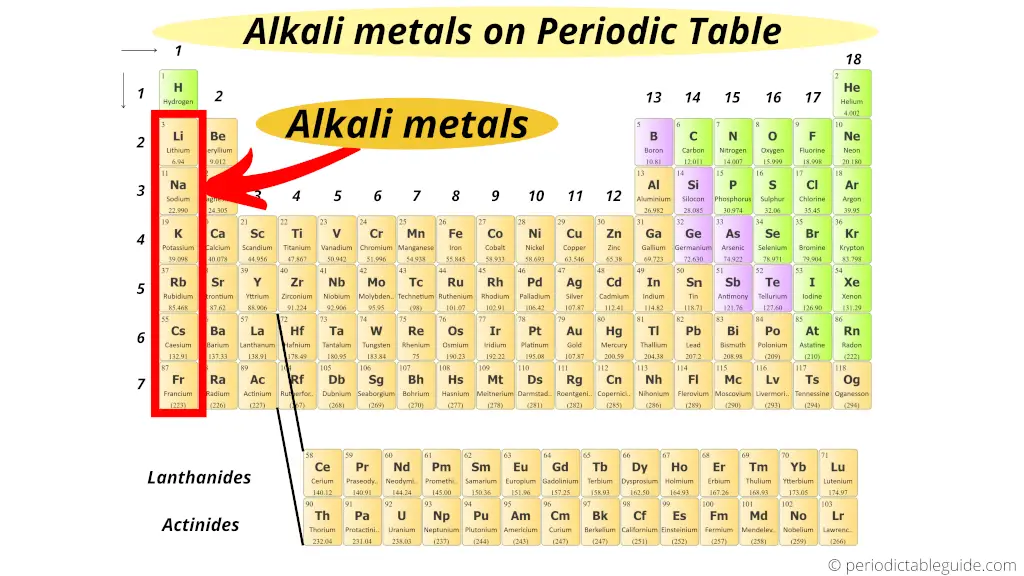
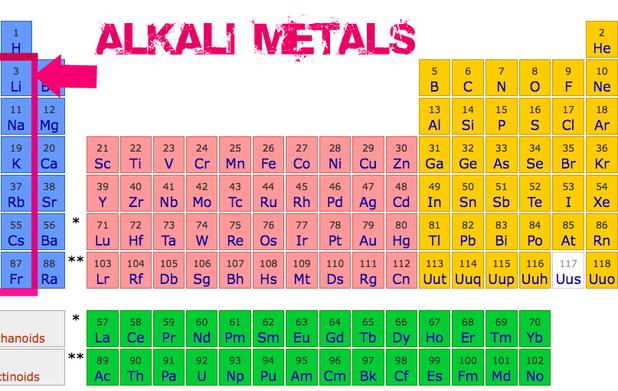
Imagine a group of friends who are always the first to arrive at the party, the first to volunteer for games, and the first to offer you a slice of pizza. That's the alkali metals in a nutshell. They're located in the very first column of the periodic table (group 1), and they're known for their extreme reactivity. These guys love to bond!
But who are these social butterflies, exactly? Let's meet the crew:
Lithium (Li): The Mood Stabilizer
First up is Lithium. You might know it as a key ingredient in batteries, powering everything from your phone to your electric car. But it's also famous for its mood-stabilizing properties, helping people feel a little more zen. Think of it as the chill friend who always knows how to calm you down after a bad day. Though, don't go licking your phone battery thinking it will make you feel better. It doesn't work that way!
Sodium (Na): The Salty Sensation
Next, we have Sodium. This is the stuff that makes your french fries taste amazing! Okay, technically it's sodium chloride (table salt), but sodium is the star of the show. It's essential for our bodies, helping with nerve and muscle function. But beware, too much sodium can lead to some serious health issues. Balance is key, my friends, balance!
Potassium (K): The Banana Booster
Ah, Potassium! This one's a vital nutrient found in bananas, sweet potatoes, and avocados (basically all the yummy stuff). It helps regulate blood pressure and keeps your heart happy. So next time you're feeling a little sluggish, grab a banana – potassium might be just what you need!
Rubidium (Rb): The Atomic Clock Master
Now we're getting into the more exotic territory. Rubidium isn't something you encounter every day, but it's used in atomic clocks, which are incredibly precise timekeeping devices. These clocks are so accurate that they can measure time to within a billionth of a second! Think about that the next time you're running late. You can't blame the clock!
Cesium (Cs): The Photoelectric Pioneer
Meet Cesium. This alkali metal has a special talent: it releases electrons when exposed to light. This "photoelectric effect" makes it useful in things like photocells and optical scanners. It's like the superhero of sensors, always ready to detect light and trigger an action!
Francium (Fr): The Fleeting Phantom
Last, but certainly not least (though arguably the most elusive), is Francium. This one is so rare and radioactive that it's practically a ghost. It exists for such a short time before decaying that scientists have only ever been able to study it in tiny amounts. It's the mysterious, almost mythical member of the alkali metal family!
Why Are They So Reactive?
So, what makes these elements so eager to react with other substances? It all comes down to their electron configuration. Each alkali metal has just one lonely electron in its outermost shell. This electron is desperate to find a partner, so it will readily jump ship and bond with another atom. This willingness to share (or, more accurately, give away) their electron is what makes them so reactive.
Imagine them as being at a school dance with only one ticket each, and being incredibly eager to get out on the dance floor to pair up with the first element that will dance with them.
A Word of Caution
Now, before you go experimenting with alkali metals in your kitchen, remember that they can be quite dangerous! They react violently with water, producing heat, hydrogen gas, and a strong alkaline solution. This reaction can be explosive, so leave the handling of these elements to the professionals.
Trust me, your sink will thank you!
The alkali metals may be a bunch of social butterflies, but they are also quite powerful. But that's what makes them so fascinating! They play crucial roles in everything from batteries to medicine to atomic clocks. They are the life of the party when it comes to chemical reactions!