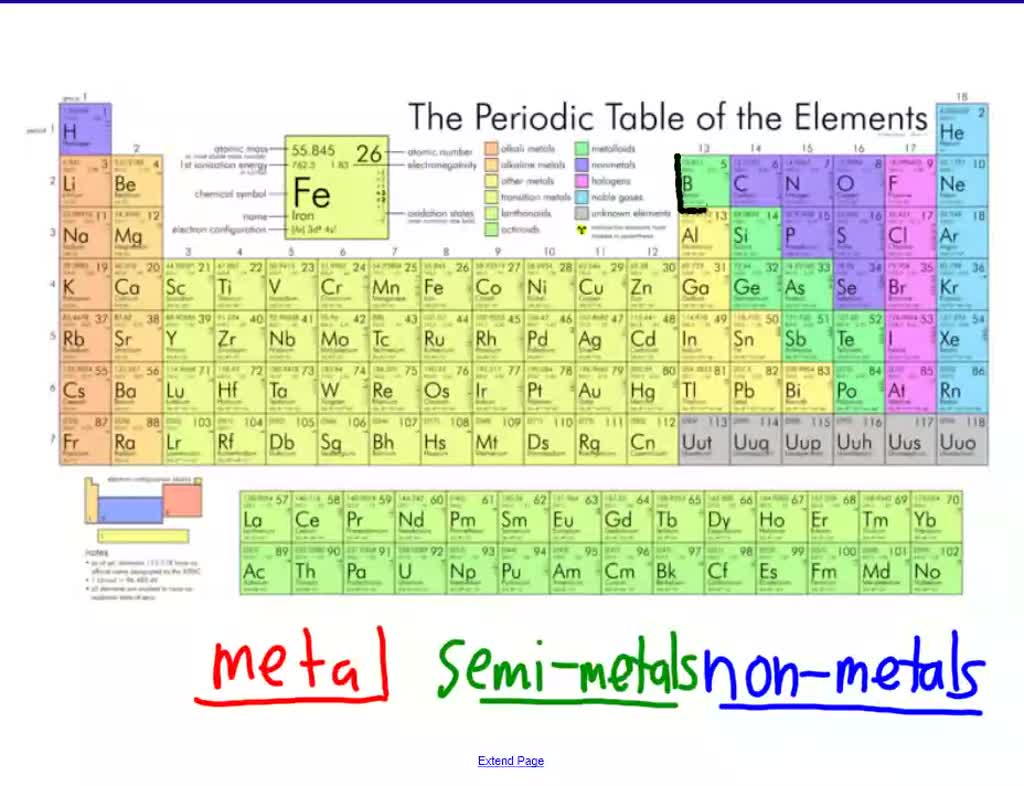
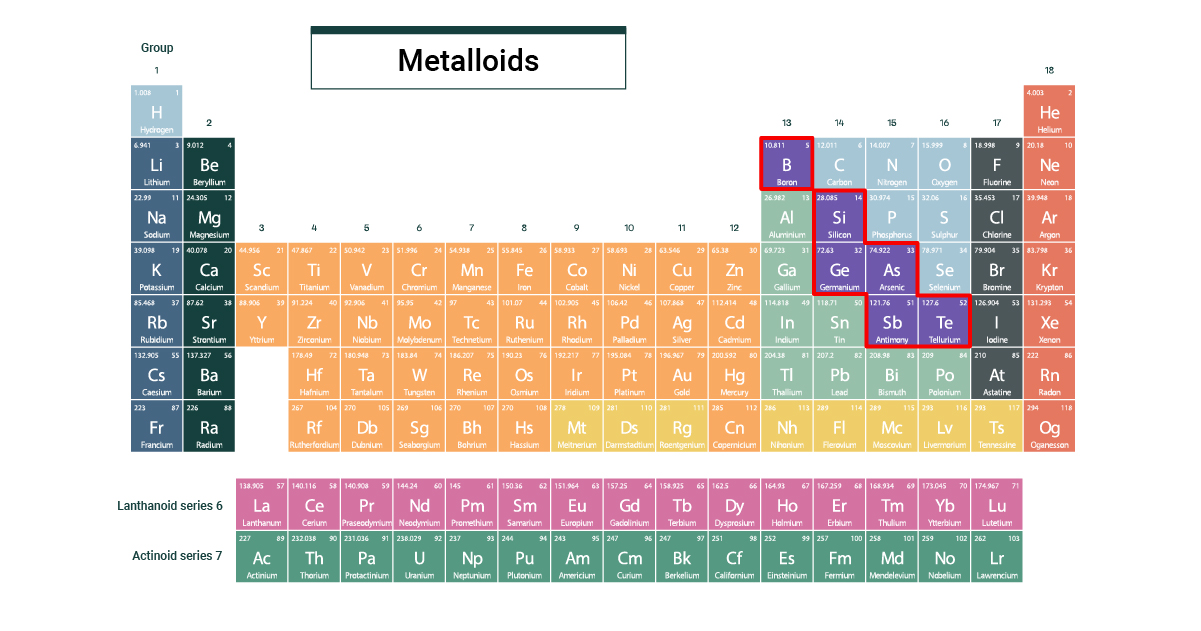
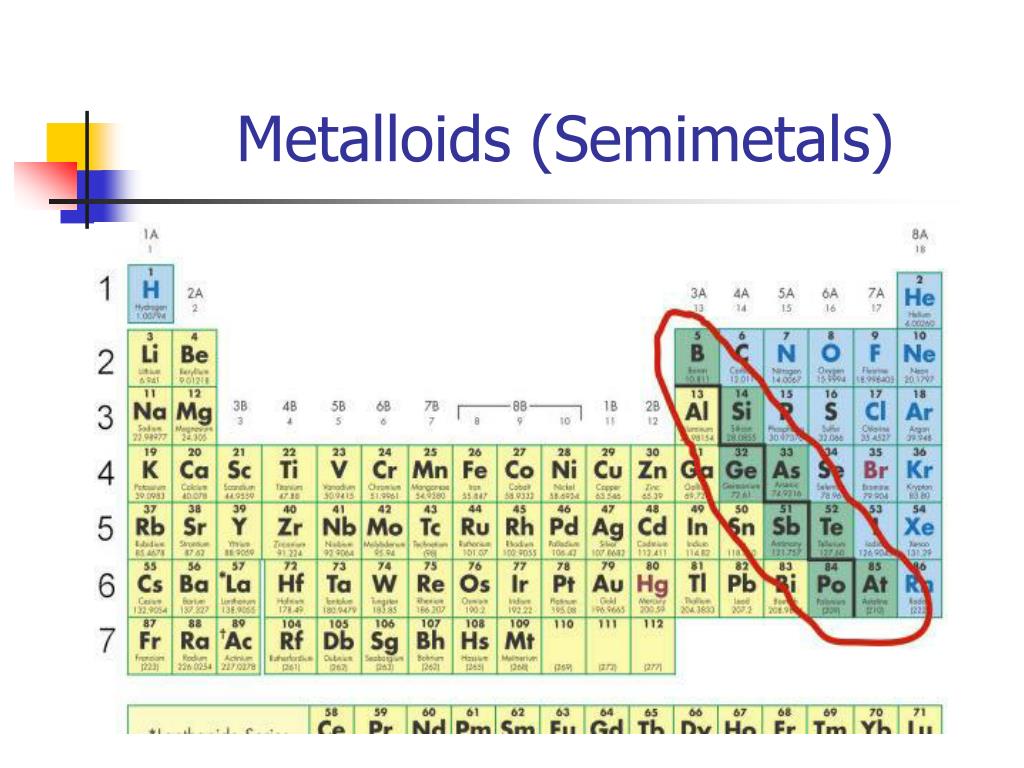
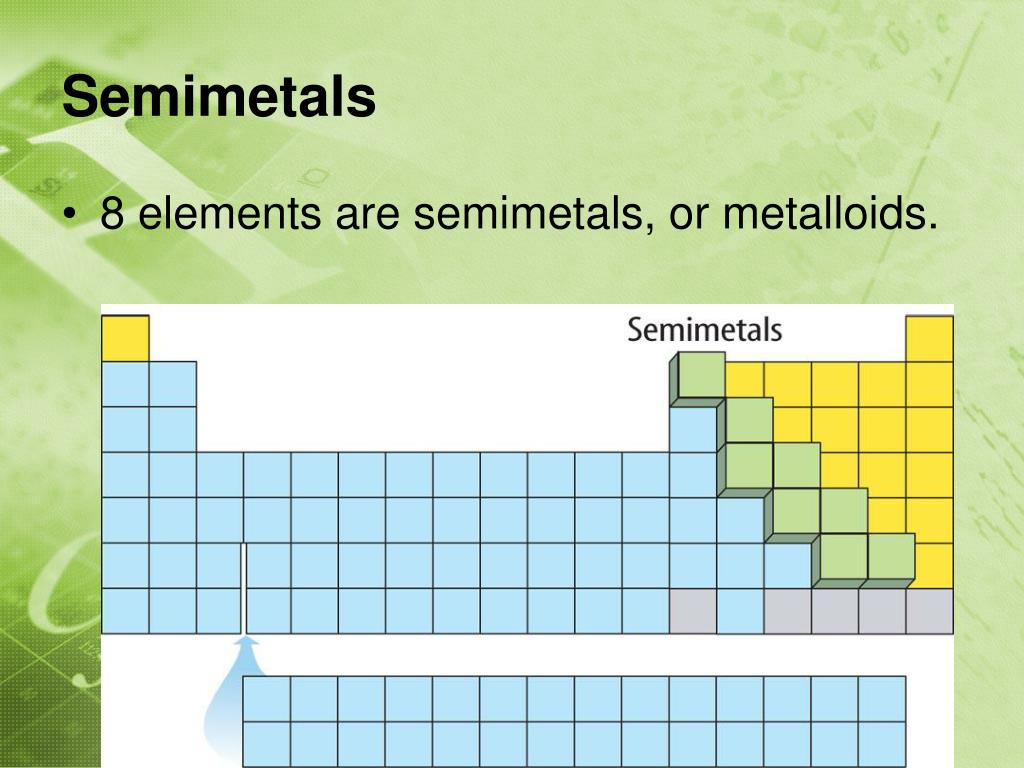
Ever looked at the periodic table and felt a little overwhelmed by all those boxes? Don't worry, most people do! But today, we're going to zoom in on one particularly interesting group: the semi-metals. Also sometimes called metalloids, these elements are like the chameleons of the element world. They're not quite metals, not quite non-metals, and that's exactly what makes them so cool and useful.
So, why should you care about semi-metals? Well, for beginners, understanding them helps you grasp the bigger picture of how elements are organized and how their properties change. Think of it as unlocking a secret level in your understanding of chemistry. For families and especially those with budding young scientists, learning about semi-metals can spark curiosity about the world around us. "Hey, did you know that the stuff in your phone has a semi-metal in it?" is a great conversation starter! And for hobbyists, especially those interested in electronics, coding, or even gardening, knowing about semi-metals can open up a world of possibilities, explaining how that computer chip works or why certain soil amendments are beneficial.
What makes these elements so special? It's all about their in-between nature. They can sometimes conduct electricity like metals, and sometimes act as insulators like non-metals. Think of it like a dimmer switch on a light. Metals are always "on," non-metals are always "off," but semi-metals can be adjusted. Some common examples include silicon, used in virtually every computer chip; germanium, another crucial semiconductor; arsenic, sometimes used in wood preservatives (though handled with care!); and boron, a key ingredient in things like borax soap and certain types of glass.
Variations in semi-metal behavior depend on factors like temperature, pressure, and the presence of other elements. Silicon, for example, becomes a better conductor of electricity as its temperature increases. This sensitivity makes it ideal for use in transistors and other electronic components. Boron's role in strengthening glass highlights another side of its versatility – it can change the physical properties of materials.
Getting started learning more about semi-metals is surprisingly easy! Here are a few simple tips:
- Explore interactive periodic tables online: Many websites offer clickable periodic tables with detailed information about each element.
- Watch educational videos: YouTube is a treasure trove of engaging content explaining the properties and uses of semi-metals. Search for "semi-metals explained" or "metalloids."
- Look around your house: Try to identify objects that contain semi-metals. Your phone, computer, even some fertilizers, likely contain them.
- Do simple experiments (with adult supervision!): Grow plants with and without boron-containing fertilizer to see the difference.
Understanding semi-metals might seem like a small detail, but it unlocks a deeper understanding of the world around you. From the technology that powers our lives to the plants that sustain us, these fascinating elements play a crucial, often unseen, role. So, dive in, explore, and enjoy the fascinating world of semi-metals! You might just discover a new passion for science and the elements that make up everything.