Let's talk about the "poor metals"! Now, that might sound like we're feeling sorry for some elements on the periodic table, but it's actually a fun and useful category to explore. They're not quite as flashy as their metal brethren, but these elements play incredibly important roles in our everyday lives. Understanding them can be surprisingly enlightening, whether you're a curious beginner, a family looking for science-related fun, or a hobbyist tinkering with electronics.
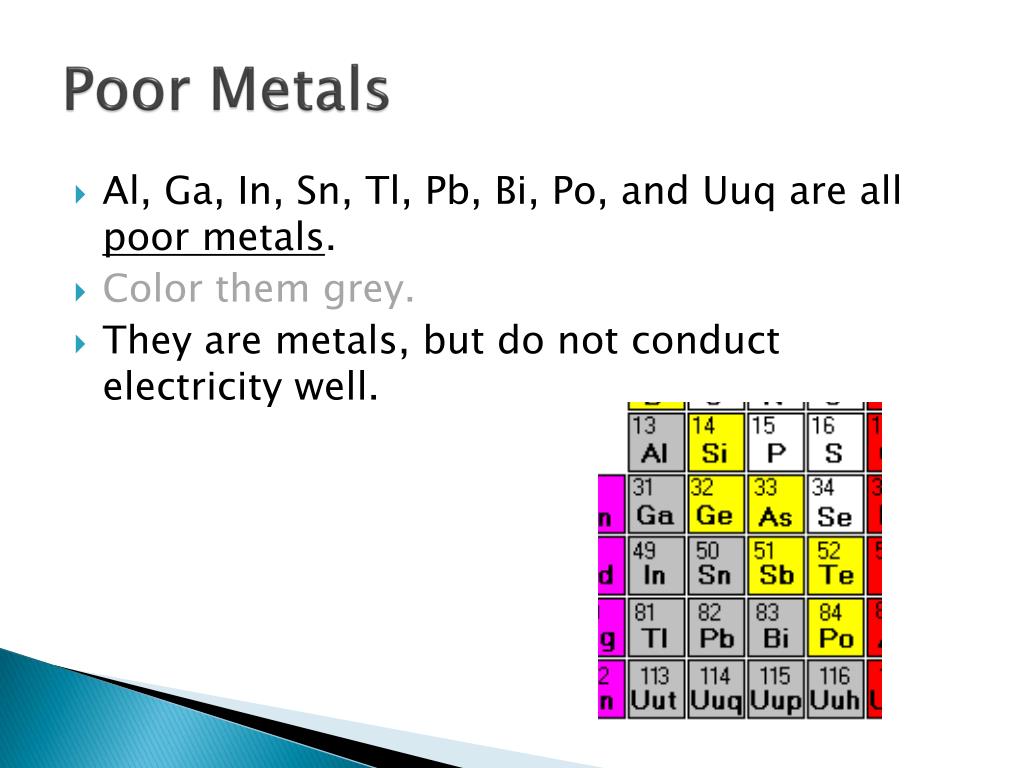
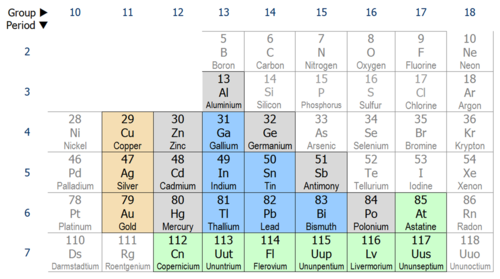
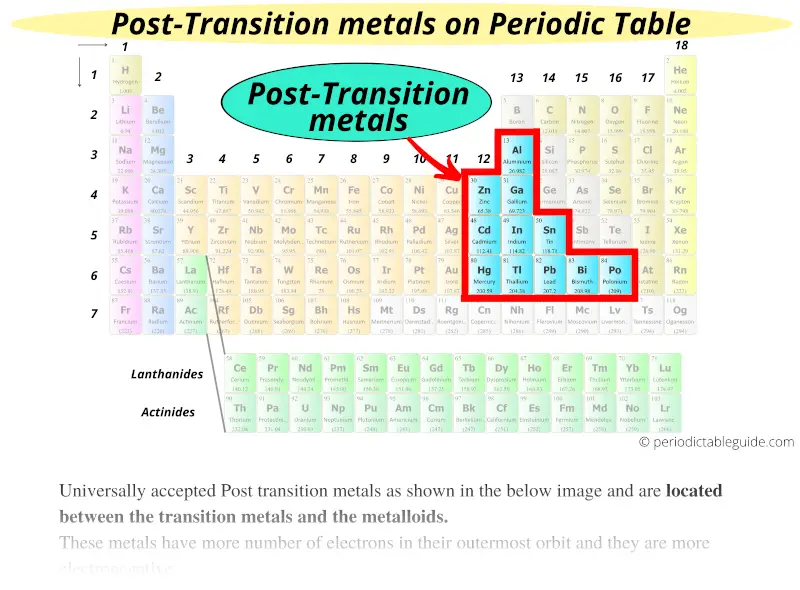
So, who are these "poor metals," and why do they matter? Officially, they're the metallic elements in the p-block of the periodic table – that's aluminum (Al), gallium (Ga), indium (In), tin (Sn), thallium (Tl), lead (Pb), and bismuth (Bi). While still exhibiting metallic properties like conductivity, they're not quite as "metal-y" as the transition metals. They're generally softer, have lower melting points, and are more likely to form covalent bonds. Think of them as the slightly more refined, sensitive metals.
For beginners, understanding the poor metals is a great way to grasp the nuances of the periodic table. It shows that being a "metal" isn't a one-size-fits-all definition. For families, exploring poor metals can be a fun science adventure. Consider making a simple alloy, like melting tin and lead to create solder (under adult supervision, of course!). You can discuss how the properties change when metals combine. For hobbyists, especially those into electronics, these metals are indispensable. Tin is a crucial component of solder, used to connect electronic components. Gallium is found in LEDs and some semiconductors. Lead, while increasingly restricted due to toxicity concerns, has been historically important in batteries.
Let's look at some examples. Aluminum, perhaps the most familiar, is used in everything from beverage cans to airplanes, prized for its lightweight and corrosion resistance. Tin coats steel cans to prevent rusting and is a key ingredient in bronze. Lead, despite its toxicity, is still used in some specialized batteries and radiation shielding. Bismuth is a relatively non-toxic metal increasingly used in cosmetics and as a substitute for lead in plumbing. These metals show amazing variations, and it's these differences that make them useful.
Want to dive deeper? Here are some simple, practical tips:
- Start with aluminum: Observe aluminum foil. Crumple it, tear it, and try to melt it (under very controlled conditions!). See how it behaves compared to other metals you might have around.
- Explore everyday objects: Look for products containing these metals. Read the labels and learn about their applications.
- Be safe! Some poor metals, like lead and thallium, are toxic. Always handle them with care and wash your hands thoroughly after contact.
Exploring the world of poor metals reveals the incredible diversity of elements and their properties. It's a journey that highlights not only the science behind these materials but also their impact on our world. So, embrace the slightly less-than-perfect world of poor metals! There's a lot to discover and enjoy in understanding these seemingly humble, yet vital, elements.