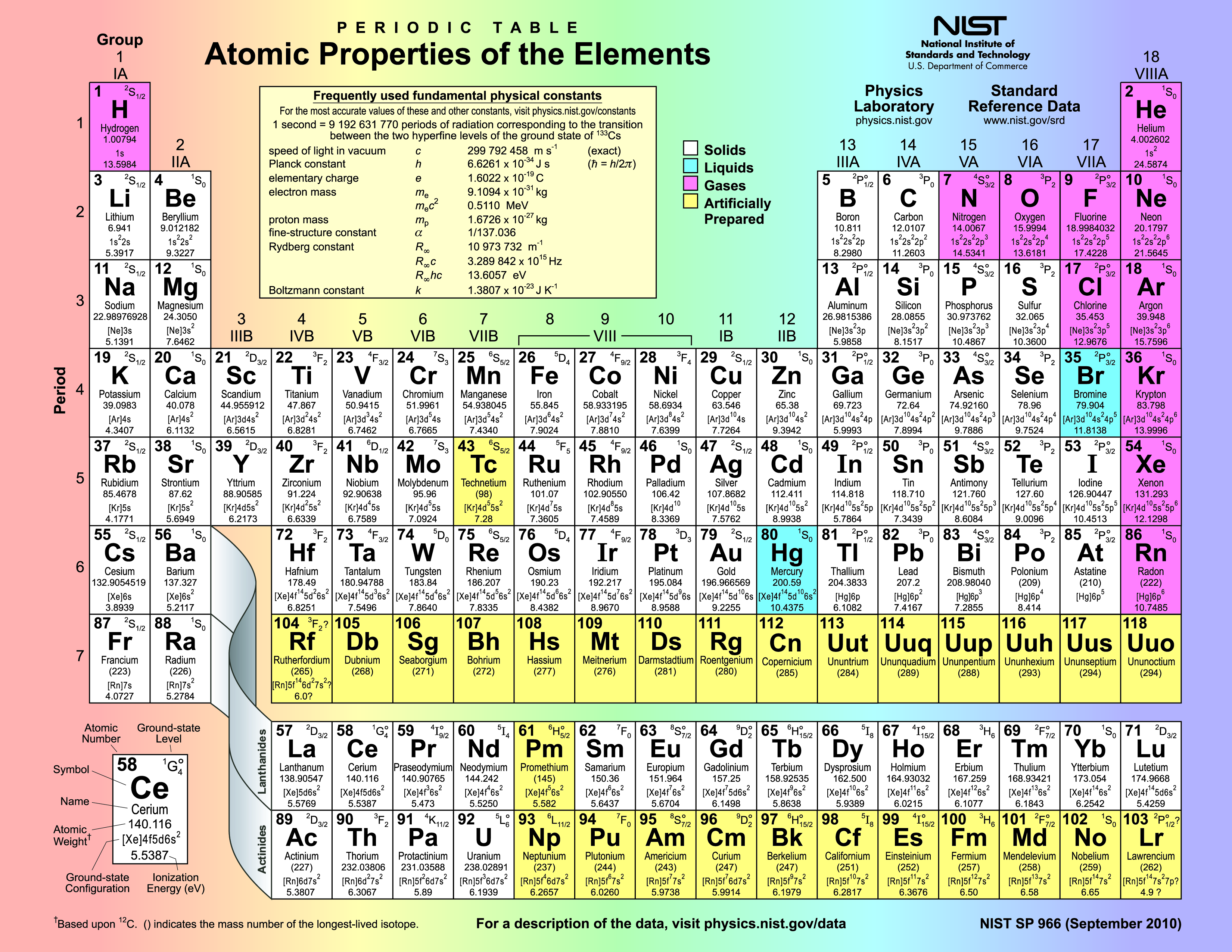
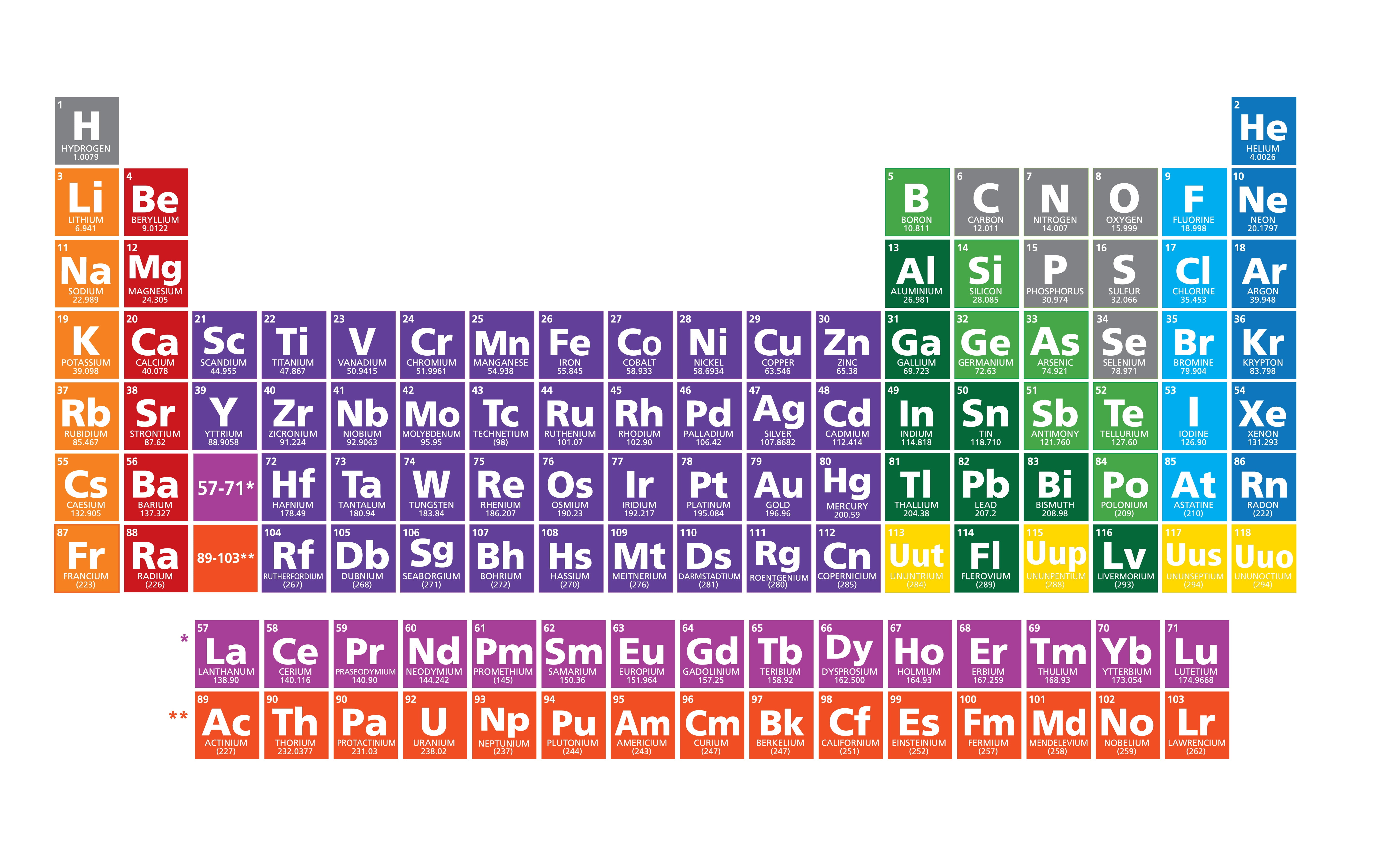
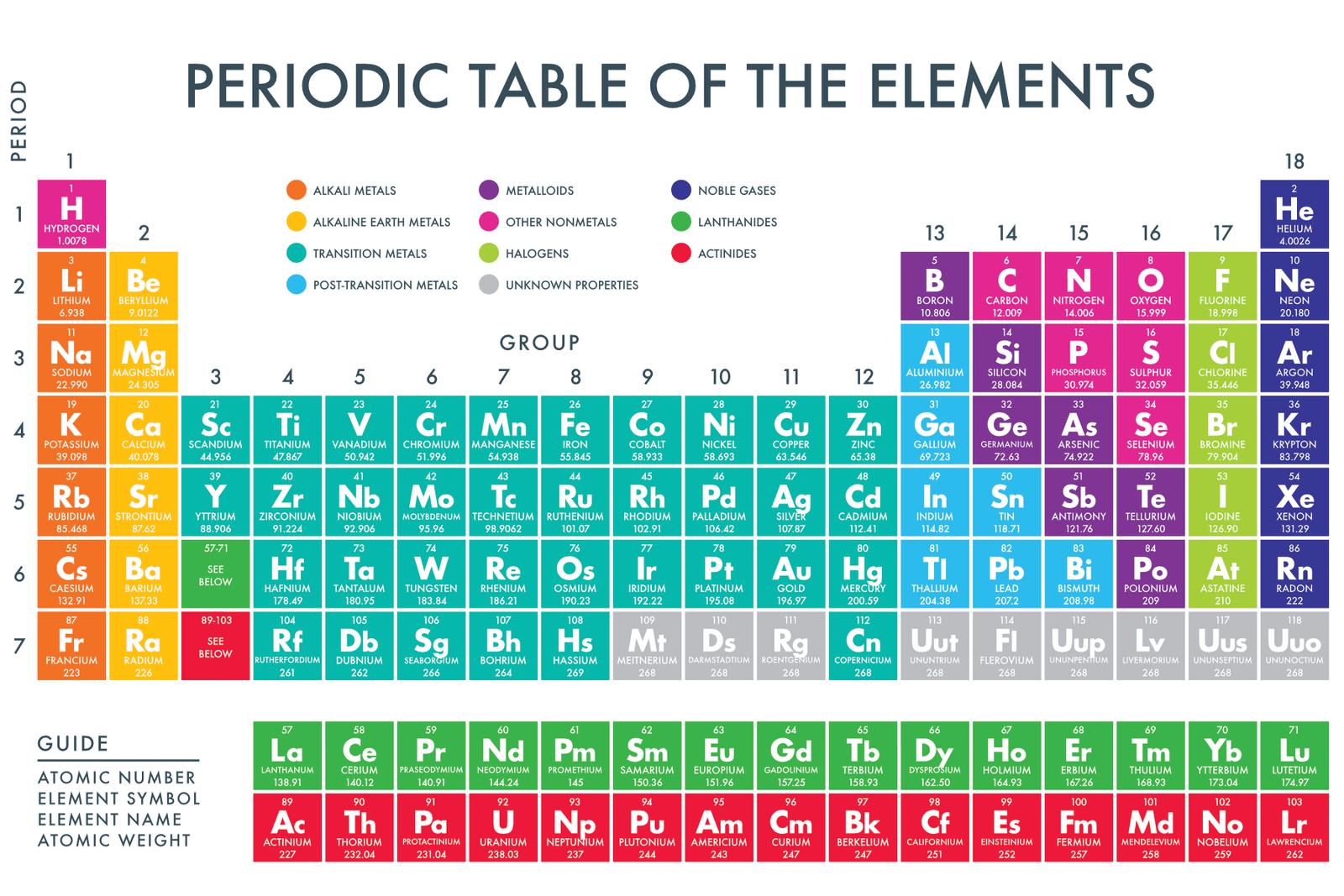
Okay, let's talk about the Periodic Table. I know, I know, it sounds like that dusty, intimidating poster hanging in your high school chemistry lab. But trust me, it's not as scary as it looks. Think of it like a seating chart for the universe's building blocks – the elements! And today, we’re going to figure out who sits where, specifically focusing on the cool kids (metals), the quirky ones (nonmetals), and the, shall we say, *fence-sitters* (metalloids).
Imagine the Periodic Table as a giant party. You've got different groups of people mingling, and we're just trying to figure out who hangs out with whom. Let’s start with the life of the party: the metals.
Metals: The Shiny Showoffs
Metals are like that friend who *always* has a shiny new gadget to show off. Think of your pots and pans – usually made of stainless steel (mostly iron and chromium). Strong, dependable, and they conduct heat like nobody's business, ensuring your pasta doesn't end up lukewarm. That's a metallic property at work! They are good conductors of electricity, so that's why the wires running through your house are copper.
These guys are generally located on the left side of the Periodic Table, and they are eager to give away electrons. They are all about sharing and being positive. Iron, gold, silver, copper… these are the headliners of the metallic world. Strong, lustrous, and often used in things that need to *last*, like bridges, jewelry, and, of course, your favorite silverware.
Ever wonder why your grandma's antique silverware is so valuable? Besides the sentimental value, it's because silver is a great conductor and pretty resistant to corrosion. Metals are used to make things work well and to protect them from the elements.
Nonmetals: The Oddballs
Now, let's move on to the nonmetals. They're like the artsy, eclectic crowd. They're located on the right side of the Periodic Table. They tend to *hog* electrons. These guys are less about sharing and more about taking.
Think about the air you breathe – mostly nitrogen and oxygen. Or the plastic containers you store your leftovers in – often made from carbon compounds. These are nonmetals at work! They don’t conduct electricity well (unless we’re talking about some fancy forms of carbon), and they are known for variety.
Nonmetals can be gases (like oxygen), solids (like sulfur), or even liquids (like bromine). They’re the chameleons of the element world. If metals are the strong, silent types, nonmetals are the dramatic actors, essential for life but not always the most predictable.
Metalloids: The Fence-Sitters
And finally, we get to the metalloids, also known as the semimetals. These are the diplomats of the element world, because they are in a zig-zag between metals and nonmetals, which means they have properties of both. Sometimes they act like metals, sometimes like nonmetals. They are the ones who can *adapt* to different situations.
Silicon, for example, is a metalloid that's a key ingredient in your computer chips. It can conduct electricity, but not as well as a metal. This is what makes it a *semiconductor*, and it's why your phone can do a million things at once without melting down (most of the time, anyway). Metalloids are the unsung heroes that make modern technology possible. Without silicon, your smartphone would be a fancy paperweight.
They are often used in electronics because their conductivity can be fine-tuned. Imagine them as the moderates in the element world, bridging the gap between the extremes. Germanium, arsenic, antimony, tellurium, and polonium also fall into this category.
So, there you have it! Metals, nonmetals, and metalloids – a quick tour of the Periodic Table's social circles. Next time you look around your house, try to spot the different elements at work. You might be surprised at how many of these *atomic* characters play a role in your everyday life!
The Periodic Table is more than just a chart; it's the recipe book for the universe. Understanding the basics of metals, nonmetals, and metalloids helps you understand how everything around you is put together. It's like unlocking a secret code, except instead of spies and gadgets, you're talking about atoms and molecules. Now go forth and impress your friends with your newfound elemental knowledge!