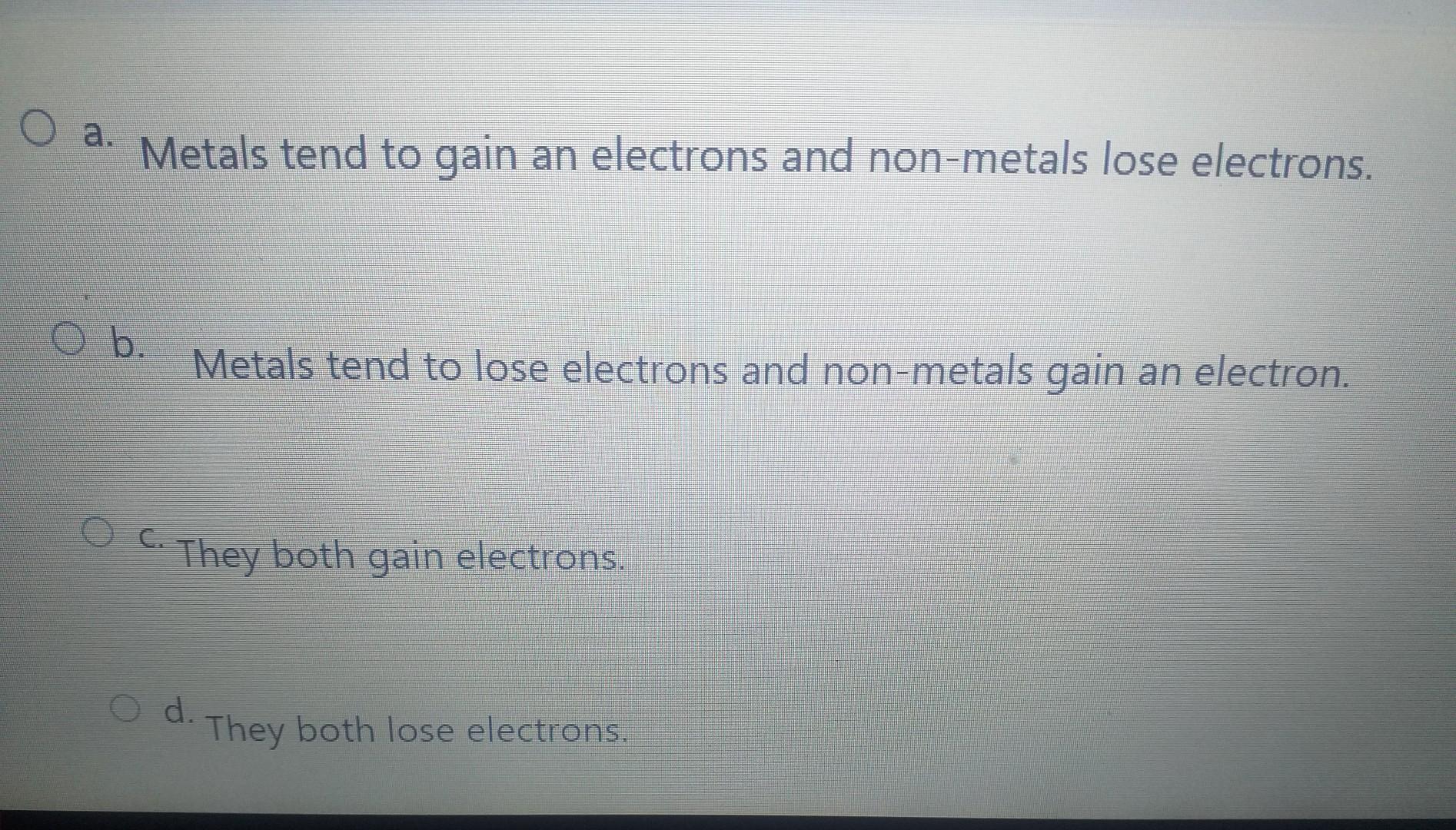
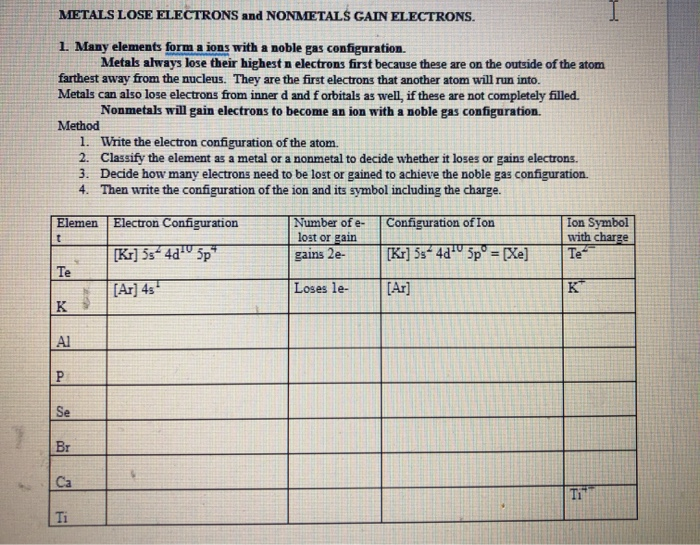
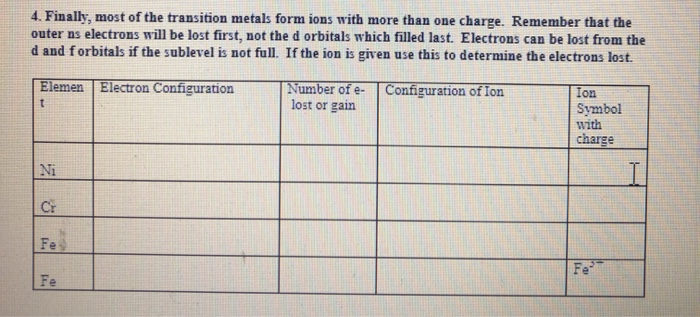
Imagine metals as the generous friends you always want around. They're the type who, when sharing a pizza, always insist you take the bigger slice, even if they're secretly craving it. In the world of atoms, that generosity translates to electrons. Metals are notorious for losing them!
Electron Giveaways: The Metal Way
Now, electrons are tiny, negatively charged particles buzzing around the nucleus of an atom like hyperactive bees around a hive. Atoms like to be stable, like a perfectly balanced seesaw. Sometimes, metals have an extra electron (or two, or three!) that throws off their balance. They're much happier when they can pass these electrons along to someone else. Think of it as getting rid of an itchy sweater – instant relief!
This eagerness to give away electrons is what makes metals so useful. It’s why we can build bridges that don’t collapse, create circuits that power our homes, and even make shiny jewelry to bedazzle our outfits. Without metals and their electron-donating tendencies, the world would be a far less connected, powered, and sparkly place. Can you imagine a world without the humble paperclip? Such a thing would bring misery to all!
So, What Do We Call These Generous Souls?
Here's the punchline! Because metals lose electrons, they are called cations! It’s a fancy word, sure, but think of it as a badge of honor. It’s their way of saying, "Hey, I'm a giver! I'm willing to part with my electrons for the greater good!"
Maybe "cations" doesn't sound particularly glamorous, but it's important in the world of science! Cations are essential in countless chemical reactions. Everything from the batteries that power our phones to the complex processes happening in our bodies relies on the movement of these electron-deficient metals.
Not All Heroes Wear Capes (Some are Just Metal)
It’s easy to take metals for granted. We use them every day without thinking about the atomic-level drama unfolding within them. But next time you pick up a penny (made of copper, a metal!), remember the humble electron. Remember the metal atom's sacrifice. Remember, in that moment of generosity, the copper transforms. Copper turns into a copper cation! It’s a silent, invisible act of kindness happening right in the palm of your hand.
So, the next time you're feeling generous, channel your inner metal. Think of those atoms willingly giving away their electrons for the benefit of everyone else. Be a cation in the world! Okay, maybe don't literally give away your electrons (we need those!), but strive to be giving and helpful like your favorite metal. After all, isn't that what true friendship (and chemistry!) is all about?
"Metals: The original electron philanthropists!"
And remember, it’s not just about losing electrons. It’s about what happens after. Those lost electrons go on to form bonds with other atoms, creating new compounds and making the world a more interesting (and functional) place. So, raise a glass (preferably one that isn’t metallic, unless you like the taste of iron) to the generous metals, the unsung heroes of the atomic world, and their inherent drive to share the electron wealth.
From the tiniest microchip to the mightiest skyscraper, metals play a vital role in our lives. And it all starts with their willingness to let go, to lose those precious electrons, and become the positive ions we know and love: the cations! Now, go forth and spread the knowledge!