Okay, picture this: I'm at a party, right? And someone starts going on and on about the periodic table. Usually, I'd beeline for the snack table, but something about the way they said "alkaline earth metals" intrigued me. It sounded kinda…earthy? Like something you'd find in a witch's brew (not that I'm into that, ahem). Anyway, naturally, my first thought was: Is barium part of this cool, earth-named gang?
Turns out, that's a question a lot of people have! So, let's dive in, shall we? No lab coats required (unless you *really* want to).
The short answer? Yes. Barium is an alkaline earth metal.
But if you're anything like me, a simple "yes" just doesn't cut it. We need the juicy details. We want to know *why*! So, let's break it down.
What's an Alkaline Earth Metal Anyway?
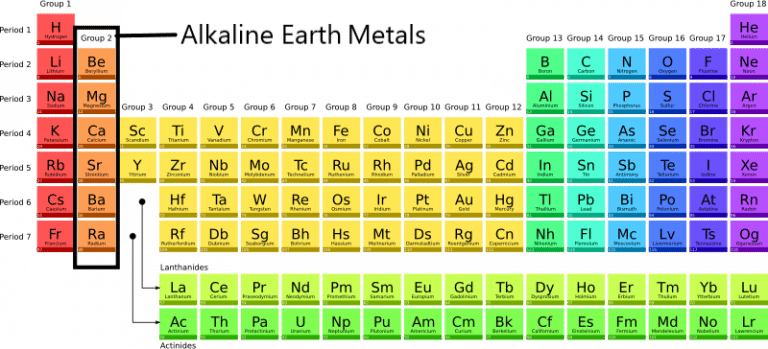
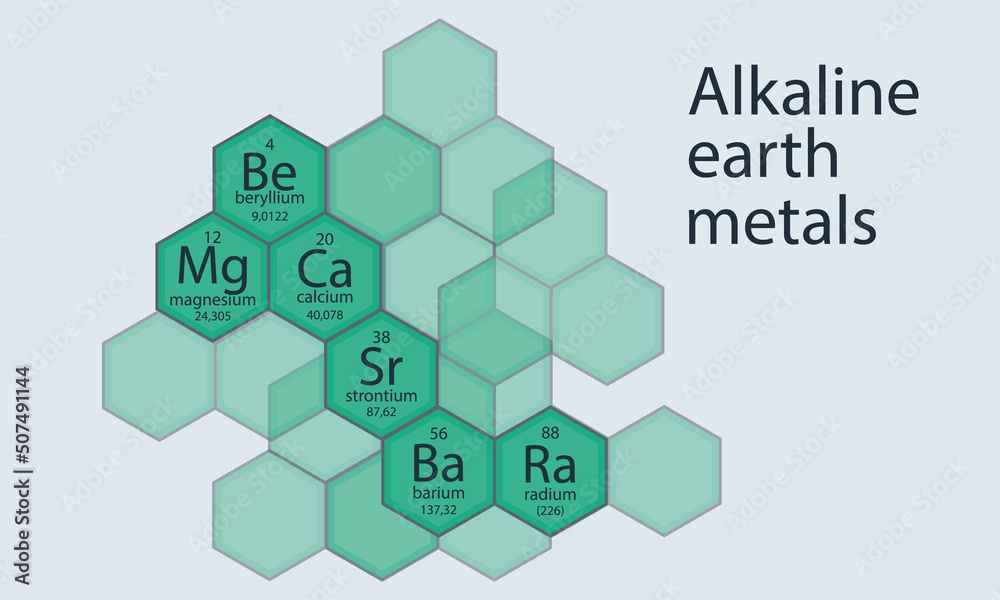
Think of the periodic table as a neighborhood, and alkaline earth metals are the residents of one particular street: Group 2. (Yep, chemists are super creative with names, aren’t they?). These guys (and gals – elements aren’t gendered, relax!) have a few things in common that make them part of the club:
- They're all metals, meaning they're generally shiny, good conductors of electricity, and like to lose electrons.
- They all have two valence electrons. That's key! It means they're always trying to get rid of those two electrons to achieve a more stable electron configuration (think wanting to be "popular" in the electron world).
- They readily form positive ions with a +2 charge (because they're losing those two electrons, remember?).
Essentially, they’re reactive metals that are *earthy* (found in the earth's crust, usually in mineral form) and form *alkaline* (basic) solutions when they react with water. Okay, maybe the name is a little more descriptive than I originally gave it credit for.
So, where does barium fit in? Glad you asked!
Barium: The Alkaline Earth Metal in Question
Barium (Ba) is sitting pretty in Group 2, right below strontium. (Periodic table neighbours, basically!). It's a silvery-white metal that's relatively soft. (You could probably scratch it… but don't! Safety first, people!). Importantly, it ticks all the boxes for being an alkaline earth metal:
- It's a metal! Obvious, right?
- It has two valence electrons. Ding ding ding!
- It readily loses those two electrons to form a Ba2+ ion.
See? Told ya! It plays by the rules of the alkaline earth metal club. And while pure barium isn't as common as some other elements (you won't find lumps of it lying around), it's found in various minerals like barite. (Fun fact: barite is used in drilling for oil and gas. Who knew!).
Barium compounds also have some interesting uses. For example, barium sulfate is used as a contrast agent in X-rays. You swallow it, and it helps doctors see your digestive system more clearly. (Suddenly, the party conversation seems a lot more practical, doesn’t it?).
Why Does It Matter?
Okay, so we know barium is an alkaline earth metal. But why should we care? (I know, the existential questions are hitting hard now.)
Well, understanding the properties of elements helps us predict how they'll behave, which is crucial in chemistry, materials science, medicine, and a whole bunch of other fields. (Suddenly wishing I'd paid more attention in high school chemistry). Knowing that barium is an alkaline earth metal tells us a lot about its reactivity, its chemical properties, and how it will interact with other elements.
Plus, it's just kinda cool to understand the world around us, right? Even if it starts with a slightly awkward conversation at a party. Now, if you’ll excuse me, all this talk about alkaline earth metals has made me hungry. Where's that snack table?
So, there you have it! Barium: Alkaline earth metal, fact. Now you can impress all your friends at your next periodic table-themed party… or just be a little bit smarter. Either way, win-win!