Hey there, curious minds! Ever wondered about the secrets hidden in your everyday objects? Like, what exactly makes that shiny spoon so... well, so spoon-y? Or why your bike frame is so darn strong?
The answer, more often than not, lies in the fascinating world of alloys! And today, we're diving headfirst into a question that might seem a bit… science-y at first, but trust me, it's way more fun than it sounds: Are alloys homogeneous mixtures?
So, grab your metaphorical lab coats (or maybe just your favorite comfy chair), and let's get started!
What's a Mixture Anyway?
Okay, before we tackle alloys, let's quickly recap mixtures. Think of it like making a salad. You toss in lettuce, tomatoes, cucumbers, maybe some croutons… each ingredient keeps its individual identity, right? That's a mixture in a nutshell: different substances physically combined.
Now, mixtures come in two main flavors: homogeneous and heterogeneous. Heterogeneous mixtures? Easy! That's our salad! You can clearly see the different parts. You can pick out a crouton (if you're feeling snacky) or avoid that one rogue tomato if you're not a fan. Homogeneous mixtures, on the other hand, are blended so well that you can't see the individual components with the naked eye. Think of saltwater – it looks just like plain water, but it's actually salt and water mixed perfectly together.
So far so good?
Alloys: The Metal Mashup
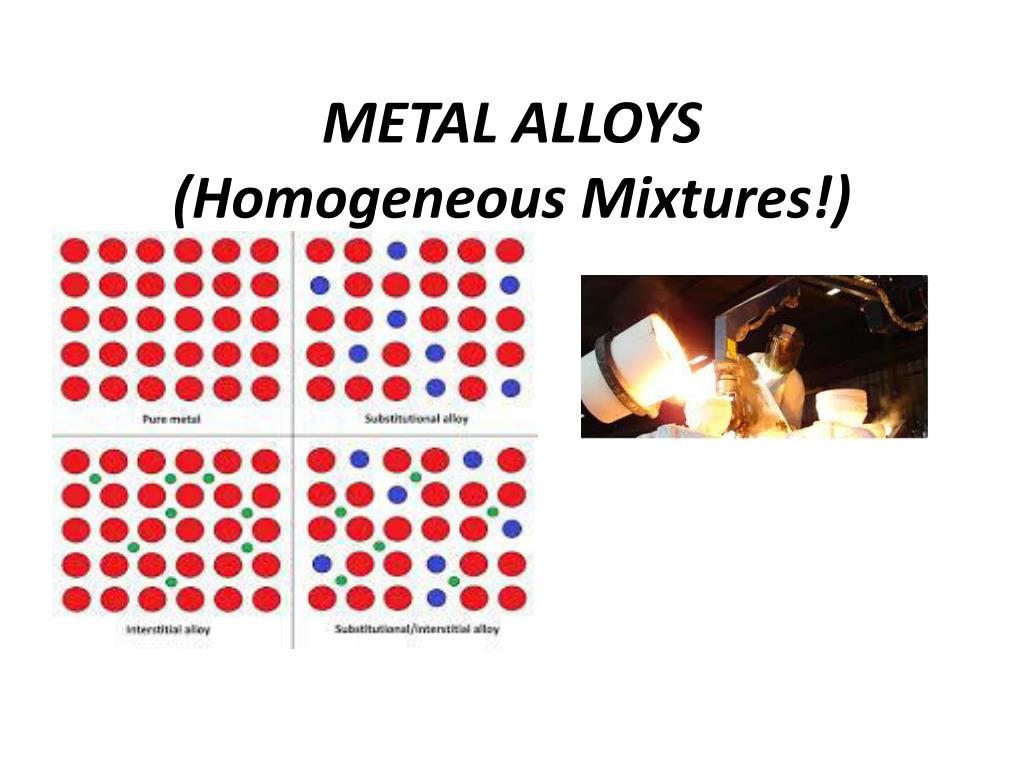
Alright, let's bring in the stars of our show: alloys! An alloy is essentially a mixture of two or more elements, where at least one of them is a metal. Think of it as a metal remix! We take different metals (and sometimes non-metals!), melt them down, and mix them together. As they cool and solidify, they form a brand-new material with properties that are often way better than the original metals on their own.
For example, pure gold is beautiful, but it's also quite soft. That's why jewelers often mix it with other metals, like copper or silver, to create stronger, more durable alloys like 14k or 18k gold. See? Alloy magic!
And stainless steel? A champion alloy! It's a mix of iron, chromium, and other elements, giving it that amazing resistance to rust and corrosion. Imagine if everything made of iron just crumbled away in the rain. No thank you!
Homogeneous or Heterogeneous? The Alloy Verdict!
Now, for the million-dollar question (okay, maybe more like a nickel question, but still important!): Are alloys homogeneous mixtures?
The answer, drumroll please… is mostly yes! In most cases, alloys are designed to be homogeneous mixtures. That's because we want the properties of the alloy to be consistent throughout the entire material. We don't want one part of your spoon to be super bendy and another part to be rock solid!
The key is that the metals are mixed at the atomic level, creating a uniform structure. You can't just look at a piece of stainless steel and pick out the iron or the chromium. They're all blended together in a beautiful, metallic harmony. It is like looking at well-mixed smoothie.
However, (and there's always a "however," isn't there?) there are exceptions! Some alloys, under certain conditions or with specific manufacturing processes, can have structures that aren't perfectly uniform. These might be considered more like heterogeneous mixtures on a microscopic scale. But for the most part, when we talk about alloys, we're talking about homogeneous mixtures.
Why This Matters (and Why It's Awesome!)
You might be thinking, "Okay, cool. Homogeneous mixtures. So what?" Well, understanding alloys and their properties helps us design and build all sorts of amazing things! From airplanes that soar through the sky to bridges that span vast distances, alloys are the unsung heroes of modern engineering.
Think about the next time you see something made of metal. Consider the properties it needs to have: strength, flexibility, resistance to corrosion, and so on. Chances are, it's an alloy carefully crafted to meet those specific requirements.
Learning about this also helps you understand the world a little better. Ever wondered why some jewelry tarnishes and some doesn't? Or why some tools last longer than others? It all comes down to the specific alloys that were used and how they interact with their environment. It gives you the kind of insider knowledge that makes you a hit at parties (or at least a more interesting person to talk to!).
Get Your Science On!
So, there you have it! Alloys are (mostly) homogeneous mixtures that make our lives better in countless ways. Isn't science amazing? It's not just about memorizing facts and formulas; it's about understanding the world around us and appreciating the incredible ingenuity of human innovation.
Now that you're armed with this newfound knowledge, I encourage you to explore the fascinating world of materials science even further. There's a whole universe of information out there waiting to be discovered! Read a book, watch a documentary, or just start asking "why?" about the things you see around you. Who knows, you might just discover your inner scientist and invent the next groundbreaking alloy! The future is metal...and the future is yours!