So, you're brewing a therapeutic protein, huh? Fancy! But hold on a sec, before you unleash it on the world, you gotta think about something kinda crucial: immunogenicity. Sounds scary, right? Don't worry, it's not rocket surgery (though sometimes it feels like it).
Basically, immunogenicity is all about whether your amazing therapeutic protein will cause the patient's immune system to throw a party… and by party, I mean an immune response. Not the kind with cake and balloons, more like the kind with angry antibodies and cellular armies.
Why is this important? Well, imagine your drug is supposed to, I don't know, cure the common cold (ambitious!). But instead, the body goes, "Whoa, what's this alien thing? Attack!" Suddenly, your drug is neutralized, and you might even have worse problems. Think: side effects, reduced efficacy, or even, gasp, a life-threatening reaction.
So, how do we figure out if our protein is going to cause a ruckus? That's where immunogenicity assessment comes in. It’s basically a fancy way of saying, "Let's check if the body's gonna freak out."
Testing, Testing, 1, 2, 3... Antibodies!
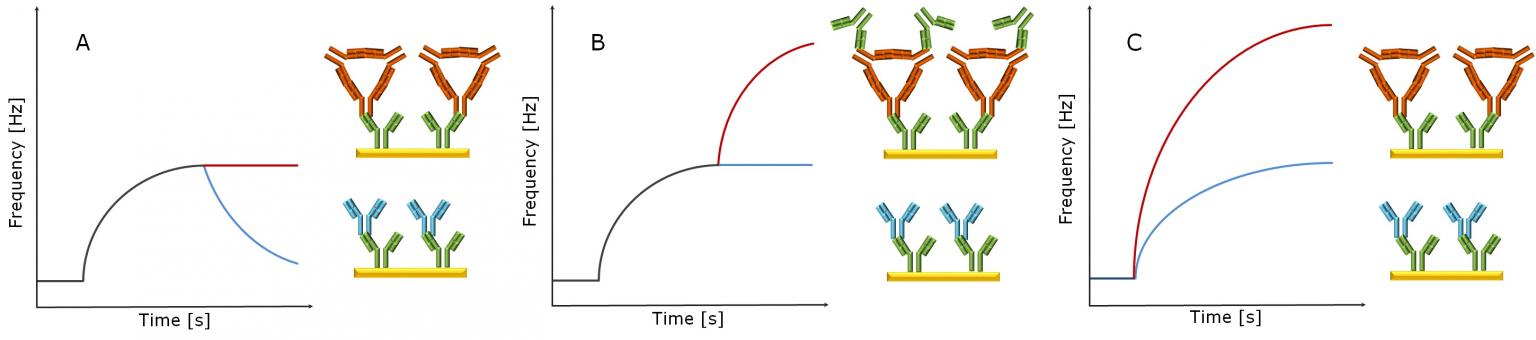
The main thing we're looking for are anti-drug antibodies (ADAs). These are the little guys the immune system creates specifically to target and neutralize your therapeutic protein. Sneaky, right?
How do we find them? Well, with assays, of course! Lots of assays. Think of it like a detective searching for clues. We use different tests to screen for ADAs, confirm they're really there, and then characterize them. Are they neutralizing? Are they binding? Are they just being annoying?
Screening assays are the first line of defense. They’re designed to be super sensitive, casting a wide net to catch any potential ADA. Think of it as a TSA agent at the airport. They're looking for anything suspicious.
But sometimes, those sensitive screening assays give us false positives. Oops! That's where confirmatory assays come in. They're like a second opinion, making sure that the ADA is actually real and not just a figment of the assay's imagination.
And then, the fun part: characterization assays! These are the Sherlock Holmes of the immunogenicity world. They delve deeper, figuring out what kind of ADAs we're dealing with. Are they neutralizing the drug? Are they affecting its pharmacokinetics (how the drug moves through the body)? This is crucial for understanding the potential impact of the immune response.
Factors That Make Your Protein "Risky"
So, what makes a therapeutic protein more likely to cause an immune response? Glad you asked!
First off, the protein itself. Is it similar to anything already in the human body? The more "foreign" it looks, the more likely the immune system is to raise an eyebrow (or, you know, launch an attack).
Then there's the dose and frequency. Think of it like this: a little sprinkle of something might be fine, but a massive dump truck load? That's gonna get noticed! The higher the dose and the more often you give it, the greater the chance of triggering an immune response.
And let's not forget the patient! Everyone's immune system is a little different. Some people are just more prone to developing ADAs than others. Genetics, underlying conditions, and even other medications can all play a role.
Why All The Fuss?
Okay, okay, I know this all sounds like a lot of work. But trust me, it's worth it! Understanding the immunogenicity of your therapeutic protein is crucial for patient safety and the success of your drug. Nobody wants their wonder drug to turn into a medical mystery, right?
By carefully assessing the immunogenicity, you can identify potential risks, develop strategies to mitigate them, and ultimately, bring safer and more effective therapies to patients. And that, my friend, is something worth celebrating (with cake and balloons, maybe?).
So, next time you're sipping your coffee and thinking about your therapeutic protein, remember: don't forget about immunogenicity! It's the key to unlocking a successful and safe drug.