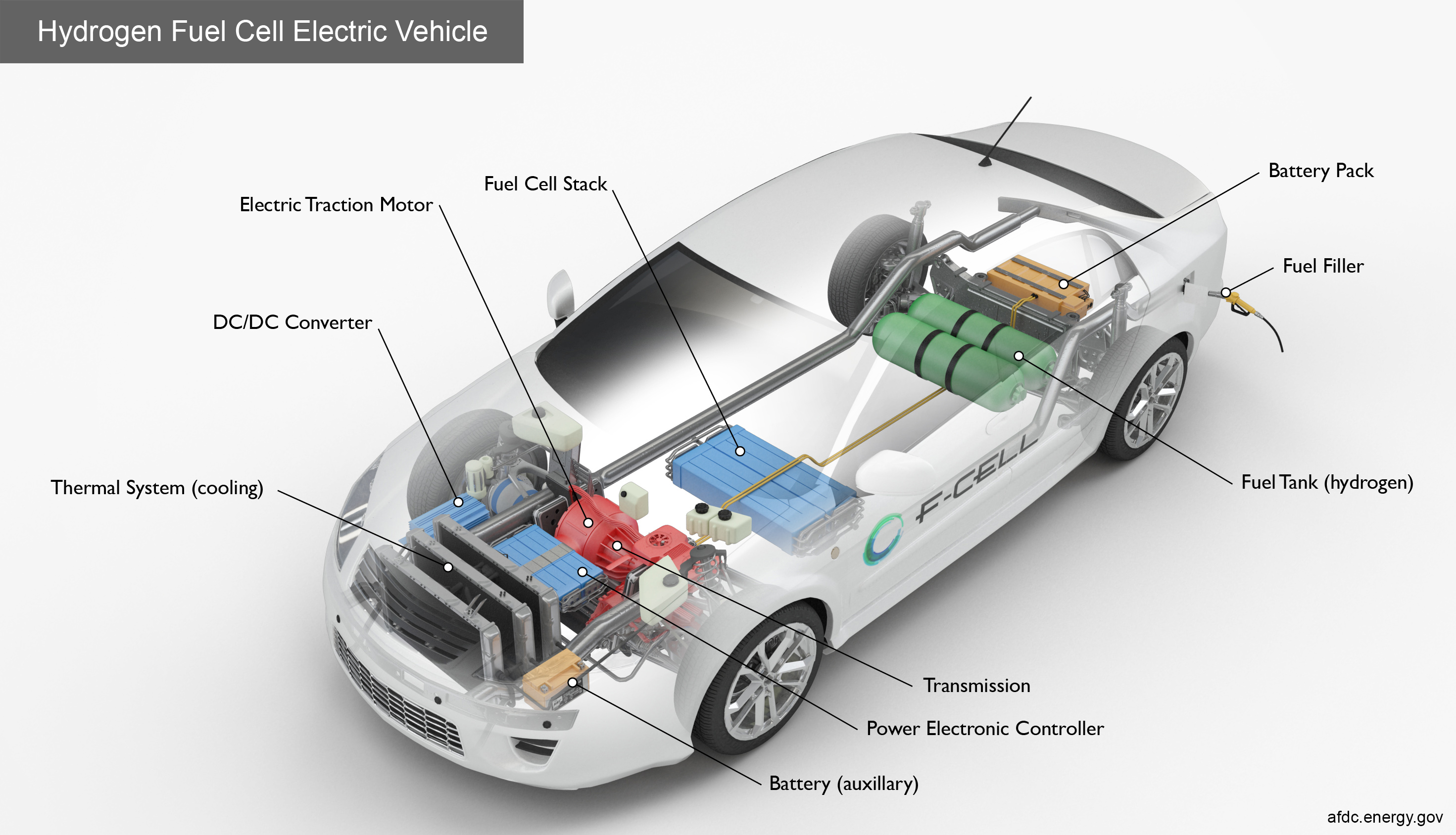
So, you want to be a hydrogen gas tycoon, huh? Fueling your car with the stuff dreams (and sci-fi movies) are made of? Well, buckle up buttercup, because while we're not exactly going to be building a hydrogen refueling station in your backyard, we can definitely explore the super-simplified, giggle-inducing version of how you could make a tiny bit of hydrogen gas.
The Water-Splitting Extravaganza (at a VERY Small Scale!)
Forget giant reactors and complex chemistry for a moment. We're going back to basics – like, middle-school science fair basics. Our star player? Good ol' H2O! Yes, water. The stuff you drink, bathe in, and occasionally accidentally spill on your keyboard. Turns out, water is secretly just a team of hydrogen and oxygen atoms hanging out together. Our mission, should we choose to accept it, is to politely ask them to part ways.
The Electrifying Performance: Electrolysis!
The magic word is electrolysis! Sounds fancy, right? Don't let it intimidate you. It just means using electricity to split water. Think of it as giving those hydrogen and oxygen atoms a little electric jolt that encourages them to go their separate ways. It's like breaking up a fight with a cattle prod, but, you know, with atoms. (Please don't use a cattle prod on anything other than...well, never mind).
Here's the ridiculously simplified lowdown (seriously, don't try to fuel your car with this!):
- Gather Your Supplies (aka, stuff you might already have): You'll need some water (distilled is best, because tap water has stuff dissolved in it that might muck things up), a 9-volt battery (the kind that powers your smoke detector), two wires with alligator clips (think of them as tiny electrical grappling hooks), and two pencils (sharpened at both ends!). And, oh yeah, a jar or a glass to hold the water.
- Setting the Stage (No pyrotechnics allowed!): Fill the jar with water. Then, carefully poke the pencils through a piece of cardboard or stiff plastic (like the lid of a margarine container). The pencils will be our electrodes – fancy word for the things that conduct electricity into the water.
- Hooking Up the Band (Safety first!): Attach one alligator clip to the positive (+) terminal of the battery and the other end to one of the pencil leads. Attach the other alligator clip to the negative (-) terminal and the other end to the other pencil lead. Submerge the pencil leads into the water. Make sure the clips on the battery are not in contact with the water! We're going for controlled separation here, not electrocution!
- The Big Finale (Bubbles!): Watch closely! You should see tiny bubbles forming on the tips of the pencil leads. One pencil will be producing hydrogen gas, and the other will be producing oxygen. Can you guess which is which? The one connected to the negative terminal is the one producing the hydrogen!
Important Disclaimer (Like, SUPER important!):
Okay, so you've made... well, a tiny, tiny, TINY amount of hydrogen. Enough to maybe fill a very small balloon. This is NOT enough to power your car, your lawnmower, or even a really enthusiastic hamster wheel. Hydrogen gas is highly flammable! This experiment is for educational purposes only! Don't try to collect the gas and set it on fire (seriously, don't). We are doing this to learn about science, not to recreate the Hindenburg disaster.
Scaling Up (Dream Big, But Safely!)
If you're serious about using hydrogen for fuel, you're going to need a MUCH larger-scale operation. Think industrial-sized electrolyzers powered by renewable energy sources like solar or wind. This is where things get really interesting (and expensive!). Researchers and engineers are working hard to develop efficient and safe ways to produce and store hydrogen on a large scale.
So, while you won't be ditching gasoline for DIY hydrogen anytime soon, hopefully, this little experiment has sparked your curiosity about the possibilities of hydrogen as a future fuel source. And who knows, maybe one day you'll be driving a hydrogen-powered car that runs on sunshine and good intentions. Now, that would be a bright future!
Remember to always be safe and responsible while doing scientific experiments. Have fun!