Hey there, curious minds! Ever wonder about the tiny, zippy particles whizzing around inside, well, everything? I'm talking about electrons! These little guys are the rockstars of the atomic world. But how do we even know how many electrons an atom has? Let's dive in – it's easier than you think!
Decoding the Atomic Number
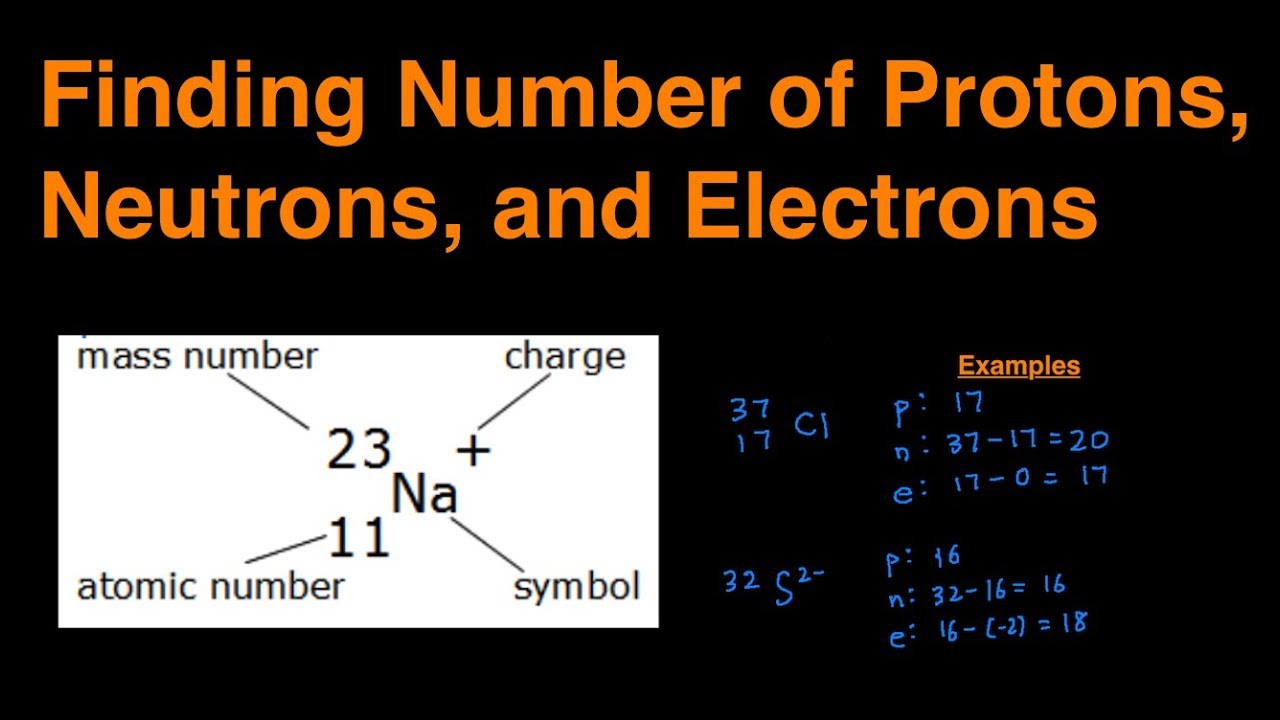
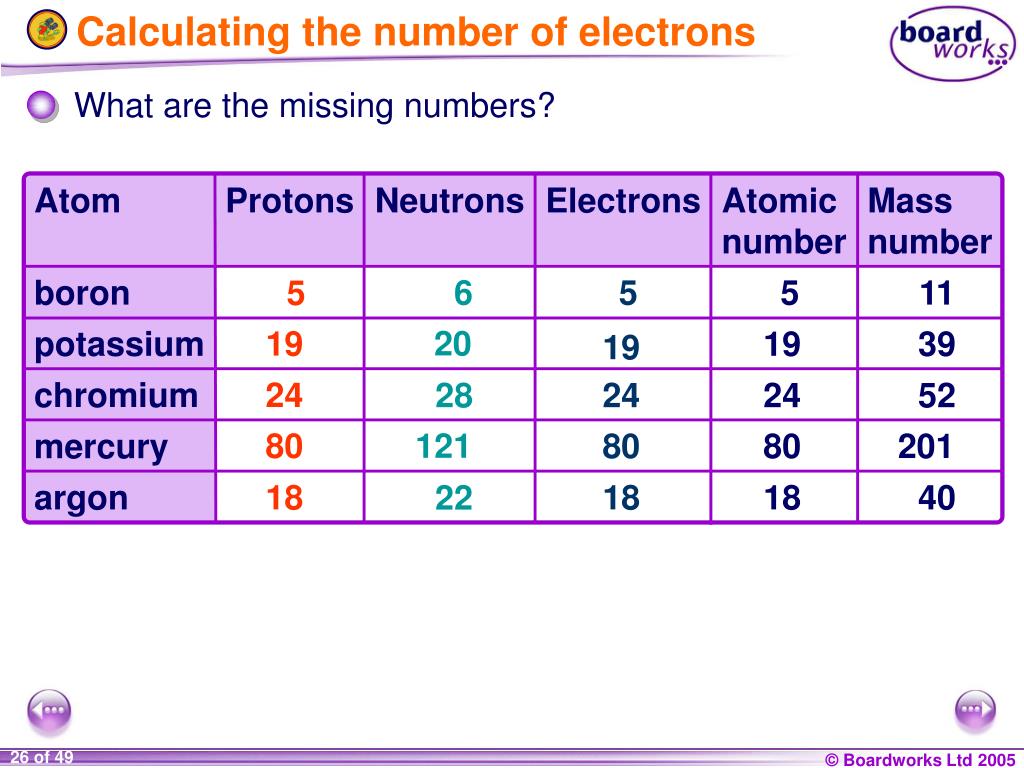
Okay, so here's the secret weapon: the atomic number. Find this number on the periodic table. It's usually chilling at the top of the element's box. This number is everything! It tells you the number of protons in the nucleus of an atom of that element. Protons are positively charged particles hanging out in the core of the atom. They are the heavy weights.
Now, here's where the magic happens. For a neutral atom (meaning it's not an ion with a charge), the number of electrons is exactly the same as the number of protons. Boom! Mind. Blown. So, if you find an element with an atomic number of 6 (that's carbon!), it has 6 protons and, consequently, 6 electrons.
Think of it like this: the atom wants to be balanced. Positives (protons) and negatives (electrons) need to be equal for the atom to be all chill and stable. Too many or too few electrons and you end up with an ion - an atom with an electrical charge. And charged atoms are like the rebels of the atomic world!
Peeking at the Periodic Table: Your Electron Number Cheat Sheet
The periodic table isn't just a pretty wall decoration in your science classroom; it's your ultimate guide to electron counting! Each element has its atomic number clearly displayed. So, if you're ever in doubt, just consult the table. It's like having a cheat sheet for the universe's smallest secrets!
For example:
- Hydrogen (H) has an atomic number of 1. Therefore, it has 1 electron.
- Oxygen (O) has an atomic number of 8. Therefore, it has 8 electrons.
- Gold (Au) has an atomic number of 79. Therefore, it has a whopping 79 electrons! (No wonder it's so valuable!)
See? Easy peasy lemon squeezy. Once you know the atomic number, you've cracked the code to figuring out the number of electrons. You are now an electron-counting ninja!
Ions: When Atoms Get a Little Extra (or Lose Some)
But wait! There's a plot twist! Sometimes, atoms aren't neutral. They can gain or lose electrons, becoming ions. If an atom gains electrons, it becomes a negatively charged ion (an anion). If it loses electrons, it becomes a positively charged ion (a cation). These ions want to react and get back to the proper number of electrons.
So, how do you figure out the number of electrons in an ion? It's still pretty simple!
- For a negatively charged ion (anion), add the number of electrons gained to the atomic number. For example, if oxygen (8 protons) gains two electrons, it becomes O2- and has 10 electrons (8 + 2 = 10).
- For a positively charged ion (cation), subtract the number of electrons lost from the atomic number. For example, if sodium (11 protons) loses one electron, it becomes Na+ and has 10 electrons (11 - 1 = 10).
See that little superscript number with a plus or minus sign? That's the charge! And it tells you how many electrons have been gained or lost.
Why Should You Care About Electrons Anyway?
Okay, so you can count electrons. Big deal, right? Wrong! Electrons are fundamental to everything! They are how all the atoms bond together. The give all materials its properties!
Think about it: electrons are responsible for:
- Chemical reactions: Electrons are exchanged and shared when chemicals react.
- Electricity: Moving electrons create electric current.
- Magnetism: Spinning electrons generate magnetic fields.
- Light: Electrons jumping between energy levels emit photons of light.
Basically, electrons are the tiny, invisible workhorses that make the world go 'round. Without them, we wouldn't have, well, anything! No chemistry, no electricity, no internet... scary thought, huh?
Go Forth and Count!
So there you have it! You are now equipped with the knowledge to figure out how many electrons an atom (or ion!) has. Grab a periodic table, impress your friends with your atomic knowledge, and remember: electrons are the tiny, zippy particles that make the universe tick. Happy counting!