Alright, gather 'round, folks! Let's talk about building a hydrogen fuel cell. Yes, you heard that right. We're gonna dive into the fascinating, slightly mad-scientist-y world of turning hydrogen into electricity. Don't worry, you don't need a PhD in quantum physics (although, if you have one, feel free to correct me!). We'll keep it simple, promise. Think of this as LEGOs for grownups… with a dash of explosive potential… okay, maybe just a sprinkle.
Now, before you start picturing yourself zipping around in a homemade hydrogen-powered car, let's be realistic. We're building a small fuel cell. Enough to maybe power a tiny LED or, if you're feeling ambitious, a very, very small robot that only knows how to say "Hello World." Baby steps, people, baby steps.
The Basic Ingredients (and Why They're Important)
Think of this like baking a cake, except instead of sugar and flour, we're using protons and electrons. Yum! Here's what you'll need:
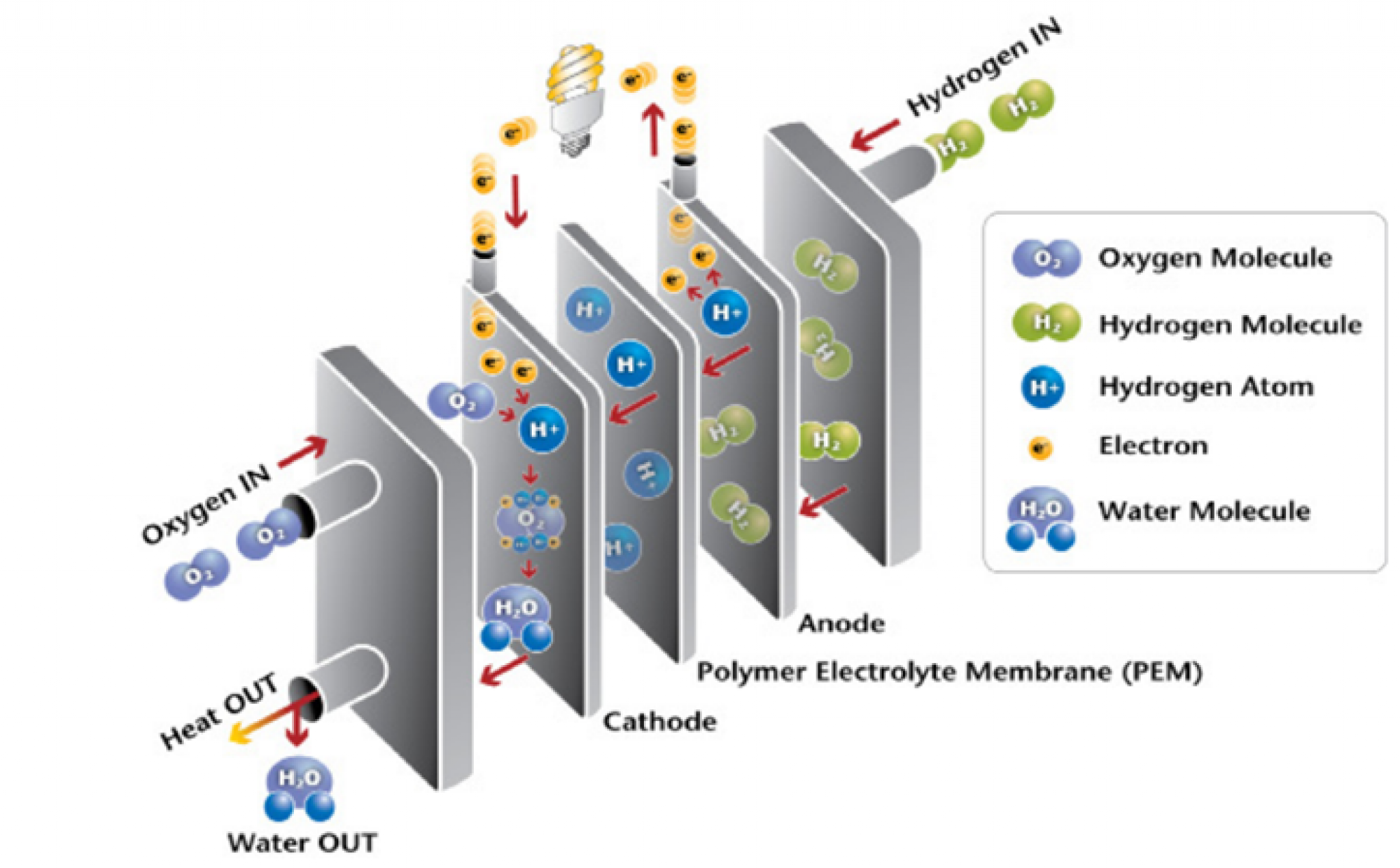
- Anode (Negative Electrode): This is where the hydrogen gets all excited and starts splitting into protons and electrons. Think of it as the hydrogen's personal rave. Usually made of a porous material coated with a catalyst like platinum. (Platinum! Fancy!)
- Cathode (Positive Electrode): This is where the oxygen hangs out, waiting to react with the protons and electrons and create... water! The end product is H2O, which is basically water.
- Electrolyte Membrane (PEM): This is the VIP section. It only allows protons to pass through, forcing the electrons to take the scenic route (i.e., through an external circuit, creating electricity!). It's super important the membrane is good quality, or else you'll short circuit your cell. Not like that'll cause an explosion, but it means you won't get any electricity.
- A Catalyst: Like a party planner, it gets the hydrogen and oxygen reacting. Usually it's Platinum! Isn't that cool?
Important Safety Note: Hydrogen is flammable. Oxygen helps fire. So... yeah, don't do this next to an open flame or a pile of dynamite. Common sense, folks. Use eye protection, and if anything starts to smell like burnt toast, back away slowly and maybe call a grown-up.
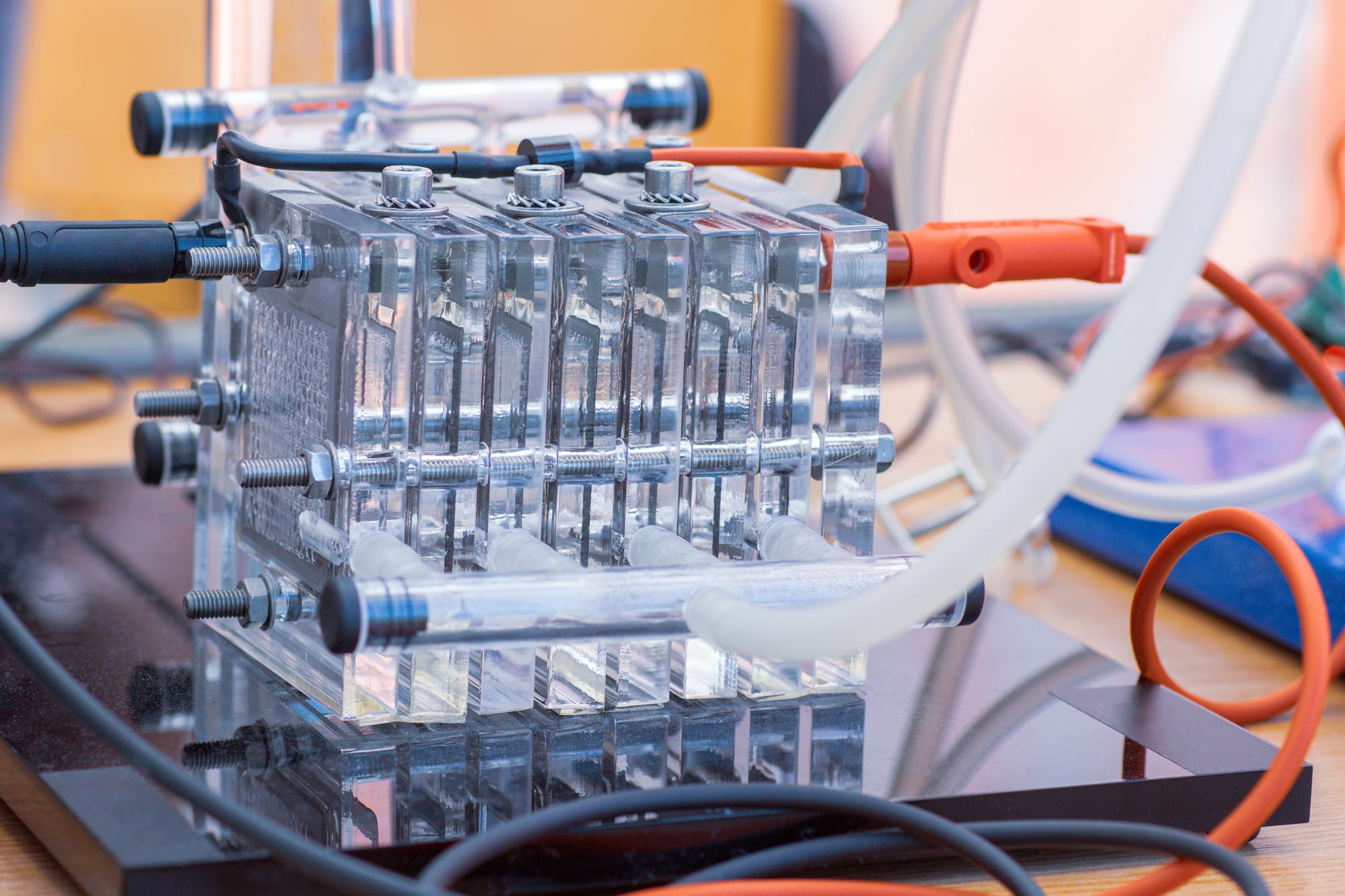
Building Your Fuel Cell: The Fun Part (Maybe)
Alright, let's get down to brass tacks. Here's a super simplified, slightly cartoonish version of how to build your fuel cell:
- Assemble Your Electrodes: Carefully attach the platinum catalyst to your anode and cathode materials. Pretend you are building a Lego castle. Be precise and avoid mistakes.
- Sandwich Time: Place the electrolyte membrane between the anode and cathode, like the filling in a hydrogen-powered sandwich. Make sure the contact is good; you don't want any proton rebellion.
- Seal It Up: You'll need to create a way to feed hydrogen to the anode and oxygen (or air) to the cathode. This usually involves some sort of sealed enclosure with gas inlets and outlets. Think Tupperware, but slightly more sophisticated. You can buy dedicated parts for this, but you can also improvise with things you find in your garage... maybe. But don't use duct tape, ok?
- Connect the Circuit: Connect the anode and cathode to an external circuit with a lightbulb or a voltmeter. Now you will have electric power and the cell is ready to be used.
The "Aha!" Moment (Hopefully): If you've done everything right (and the hydrogen gods are smiling upon you), you should see a voltage reading on your voltmeter or a faint glow from your LED. Congratulations! You've just created electricity from hydrogen and oxygen. You are one step closer to replacing fossil fuels, and saving the planet! Take that, coal.
Troubleshooting: Because Things Rarely Go As Planned
So, you followed all the steps, and nothing happened? Don't panic! Here are a few common culprits:
- Bad Connections: Make sure everything is connected properly. A loose wire is the bane of every DIY project.
- Membrane Problems: If the electrolyte membrane is damaged or not properly hydrated, it won't work. Try a new one.
- Gas Leaks: Hydrogen leaking out is not good (see safety note above). Check your seals and connections.
- Impure Hydrogen: This is a big one. Your fuel cell won't work efficiently (or at all) if the hydrogen is contaminated. You need high-purity hydrogen.
Pro Tip: Google is your friend. Seriously, there are tons of resources online about building fuel cells. Don't be afraid to ask for help! And remember, even the greatest scientists had their fair share of failures. Thomas Edison didn't invent the light bulb on his first try. He just found 10,000 ways not to make a light bulb. (Probably while muttering under his breath about electrons.)
Conclusion: You Did It! (Maybe)
Building a hydrogen fuel cell is a fun and educational project that can give you a glimpse into the future of energy. It's not always easy, but the feeling of generating electricity from hydrogen and oxygen is pretty darn cool. So, go forth and experiment! Just remember to be safe, have fun, and don't blow anything up (unless it's part of a carefully controlled experiment, of course). And who knows, maybe one day you'll be the one to invent the next breakthrough in hydrogen fuel cell technology. You are now an official fuel cell enthusiast!