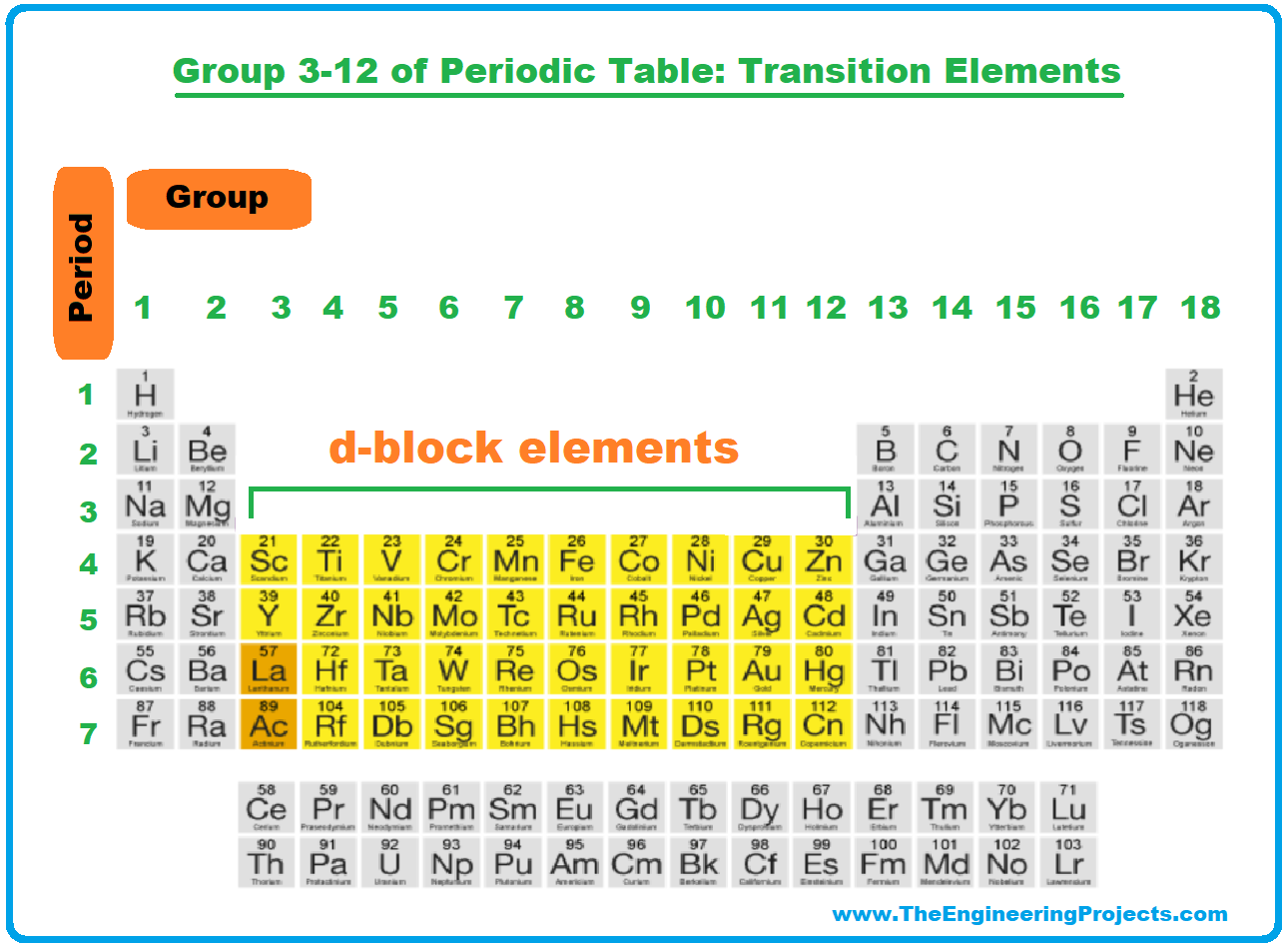
Ever felt a strange satisfaction looking at a beautifully organized bookshelf, or maybe alphabetizing your spice rack? There's a deep-seated human pleasure in structure and order, and that's a big part of why people find the periodic table so compelling. It's a map of the universe, neatly laid out, and nestled right in the heart of that map, from groups 3 through 12, lies a fascinating collection of elements known as the transition metals.
Now, you might be thinking, "Okay, cool chart, but what does it have to do with me?" The answer? Everything! These elements aren't just abstract concepts confined to a lab; they're the workhorses of modern society, silently powering our technology, strengthening our infrastructure, and even keeping us alive.
Think about it. That smartphone you're probably holding? It's packed with transition metals. Gold (Au) and copper (Cu) provide crucial electrical conductivity, while elements like tungsten (W) might be used in the vibration motor. The strong and durable steel used in bridges, buildings, and cars? Primarily iron (Fe), a transition metal through and through. Even the vibrant colors in your favorite paints and pigments often come from compounds containing transition metals like chromium (Cr) or titanium (Ti).
But their impact goes far beyond technology and infrastructure. In the realm of biology, iron is crucial for carrying oxygen in our blood. Zinc (Zn) boosts our immune systems. And manganese (Mn) plays a vital role in enzyme function. Without these elements, life as we know it wouldn't exist.
So, how can you appreciate these essential elements more effectively? Here are a few practical tips:
- Explore the stories behind the names: Many elements are named after places, scientists, or even mythological figures. Learning the origins can make them feel more real and less abstract. For instance, vanadium is named after Vanadis, the Scandinavian goddess of beauty.
- Investigate their properties: Transition metals are known for their diverse properties, including their ability to form colorful compounds and act as catalysts. Delving into these characteristics can be surprisingly engaging.
- Look around you: Start noticing where transition metals are used in your everyday life. From the stainless steel appliances in your kitchen to the rebar in a construction site, they're everywhere! This exercise can bring the abstract world of chemistry into concrete focus.
- Try simple experiments (safely!): There are many safe and easy experiments you can do at home to demonstrate the properties of transition metals. For example, you can observe the color changes of copper compounds when heated or dissolved in different solutions. Remember to always follow safety precautions and adult supervision is recommended.
Ultimately, understanding and appreciating groups 3-12 of the periodic table isn't just about memorizing names and symbols. It's about recognizing the fundamental building blocks that shape our world and understanding the profound impact of chemistry on our lives. So, the next time you see the periodic table, remember that it's more than just a chart – it's a window into the amazing and interconnected world around us.