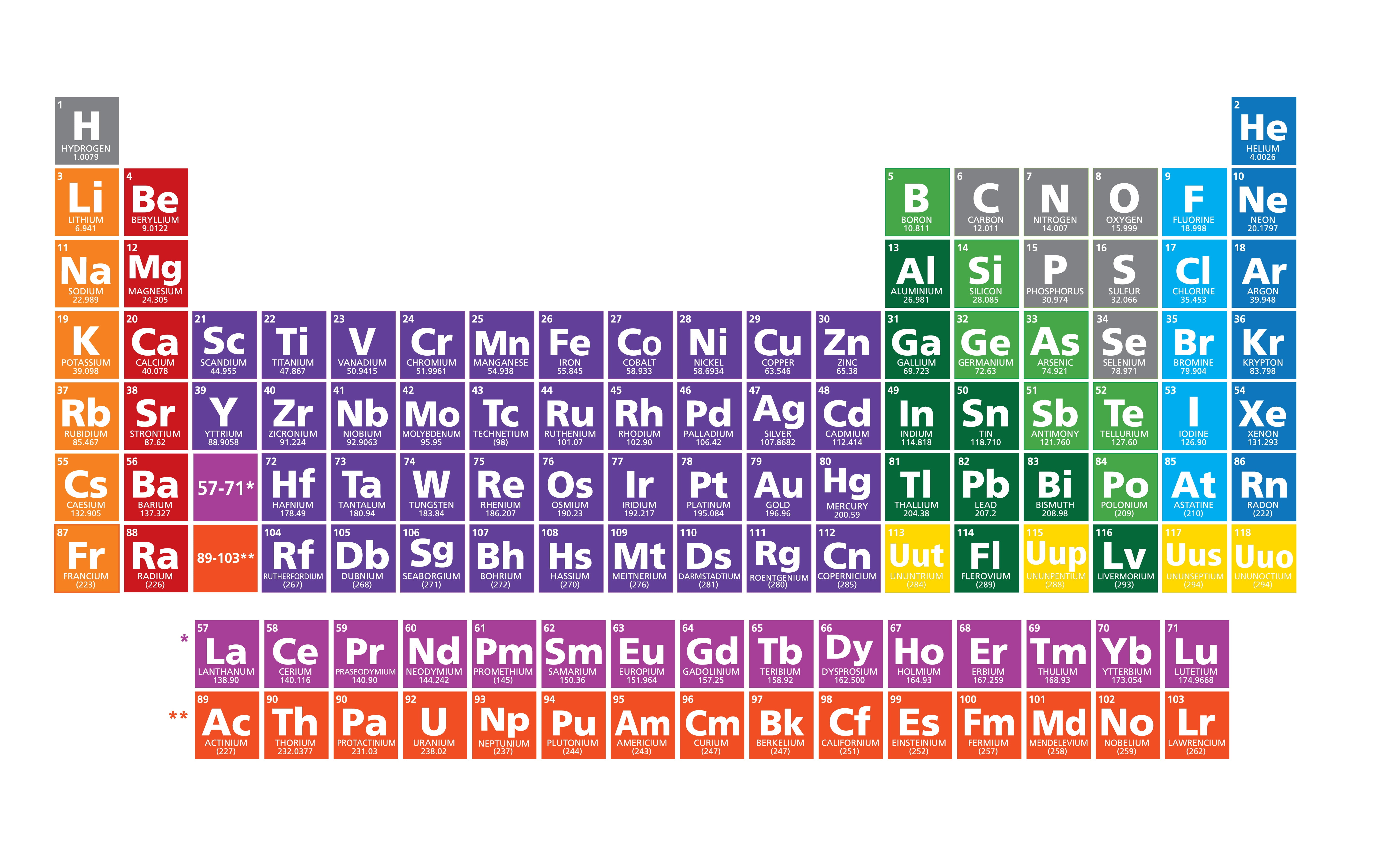
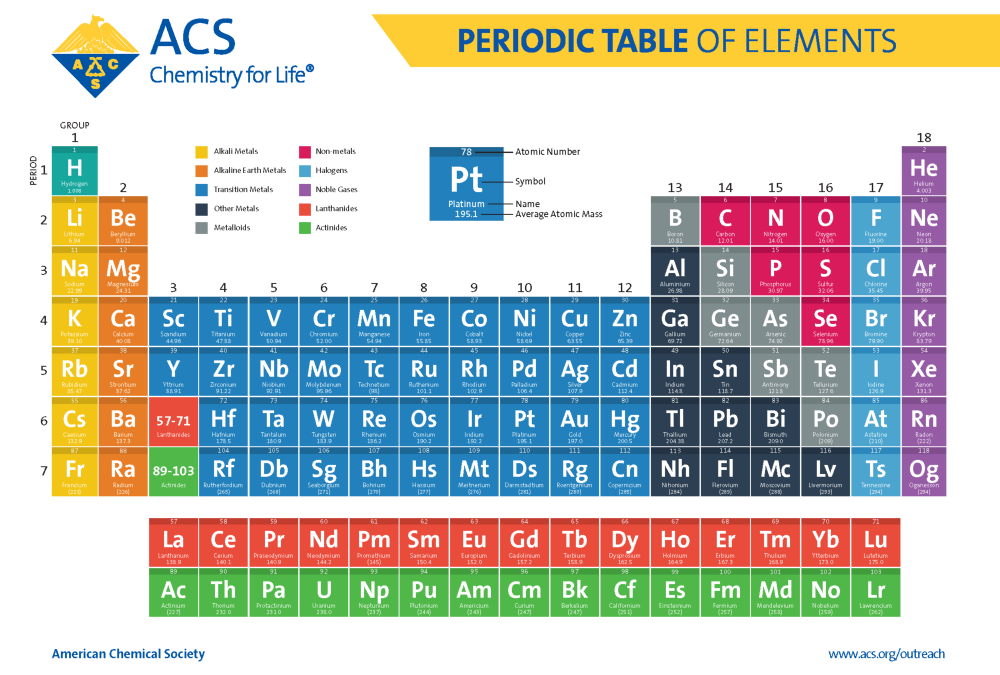
Okay, folks, let's talk about the weirdos of the periodic table. You know, the elements that just can't seem to make up their minds. Are they metal? Are they nonmetal? It's like they're stuck in a perpetual identity crisis! I'm talking about the metalloids!
What ARE These Metalloid Things?
Imagine a kid who wants to be both a superhero AND a supervillain. That's basically a metalloid. They've got properties of both metals and nonmetals. It's like they raided both camps and stole the best (and sometimes worst) qualities from each.
Think of silicon (Si). You find it in computer chips, making our phones and laptops work. That's pretty metal-like, right? But it's also a semiconductor, meaning it doesn't conduct electricity as well as a "true" metal. So, it's kind of wishy-washy about the whole electricity thing. Very nonmetal.
Then there's boron (B). It's used in stuff like laundry detergent (to help brighten your whites!). Boron compounds can be tough like a metal. Yet, it isn’t very shiny or a good conductor, which is definitely a nonmetal trait.
My (Slightly Unpopular) Opinion
Here's where I might lose some of you. I think metalloids are the most interesting elements! Hear me out.
The “true” metals are great, I get it. Shiny, strong, conduct electricity. Blah, blah, blah. We all know the drill. And the nonmetals? Okay, some of them are crucial for life (looking at you, oxygen!). But they’re often gases or brittle solids. Snore.
But metalloids? They're unpredictable! Their properties change depending on the conditions! They're the rebels of the periodic table, refusing to be pigeonholed. They're the actors who can play any role!
“Metalloids: The chameleons of chemistry.”
Examples, Please!
Let's look at germanium (Ge). It’s another semiconductor. The electrical conductivity of germanium can be altered by adding impurities. Like adding a dash of drama to an otherwise predictable performance.
And what about arsenic (As)? Okay, granted, it has a bit of a bad reputation. (Think old movies and mysterious poisons.) But in small doses, arsenic compounds have been used medicinally. See? Even the "bad boy" metalloids have a softer side!
The Metalloid Hierarchy (According to Me)
So, if I had to rank the metalloids (purely subjectively, of course), it would go something like this:
- Silicon: Because, hello, technology!
- Germanium: Always reliable and versatile.
- Boron: Helping keep our socks sparkly clean.
- Arsenic: A bit edgy.
- Antimony (Sb): Often used in alloys. Respectable.
- Tellurium (Te): Used in solar panels. Doing its part!
- Polonium (Po): It's just a bit too...radioactive. Sorry, polonium.
But honestly, they're all fascinating. They're the elements that make us think. They force us to reconsider our definitions. They're the reminder that things aren't always black and white (or metal and nonmetal!).
In Conclusion (Sort Of)
So, next time you're looking at a computer chip or reading about some scientific breakthrough, remember the humble metalloid. They're the unsung heroes, the rule-breakers, the elements that prove that sometimes, the best things in life are a little bit of both. And maybe, just maybe, being a little bit of both is the coolest thing you can be.
Now, if you'll excuse me, I'm going to go contemplate the duality of life. And maybe do some laundry. Thanks, boron!