Imagine you're throwing a party. You've got balloons, music, and a guest list packed with fun-loving molecules! But what if one guest, let's call him Hal, is being a total party pooper? He's dragging his feet, making everything move at a snail's pace. That's kind of what happens in certain chemical reactions involving haloarenes!
So, what's a haloarene? Think of it as a benzene ring (a cool hexagonal molecule – picture a honeycomb!) that's got a halogen atom – like chlorine (Cl), bromine (Br), or iodine (I) – stuck onto it. These halogens are our "Hal" from the party, and they tend to make things… slower.
The kind of party we're talking about is called an electrophilic substitution reaction. Sounds fancy, right? Don't worry! It just means that an "electrophile" (a molecule that loves electrons) is trying to crash the party and replace one of the hydrogens on our benzene ring.
Halogens: The Party Dampeners
Now, why does Hal, er, I mean the halogen, slow things down so much? Well, it's a combination of a few things, like Hal stubbornly refusing to dance or even mingle!
First, there's this thing called the inductive effect. Halogens are electron-hogging atoms. They pull electron density towards themselves through the bond connecting them to the benzene ring. This makes the benzene ring slightly less electron-rich, and electrophiles, who are looking for electron-rich environments, aren't as attracted.
Imagine you're trying to attract someone with a delicious pizza. If someone keeps stealing slices of the pizza before they can get to it, they might not be as interested! The halogen is stealing electron "slices" from the benzene ring.
Resonance: A Complicated Relationship
But wait! There's more! Halogens also have this thing called resonance going on. It's like they have a secret, hidden talent that can slightly counteract their electron-hogging ways.
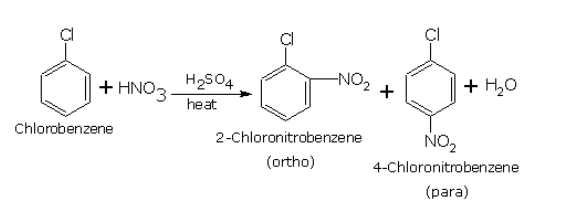
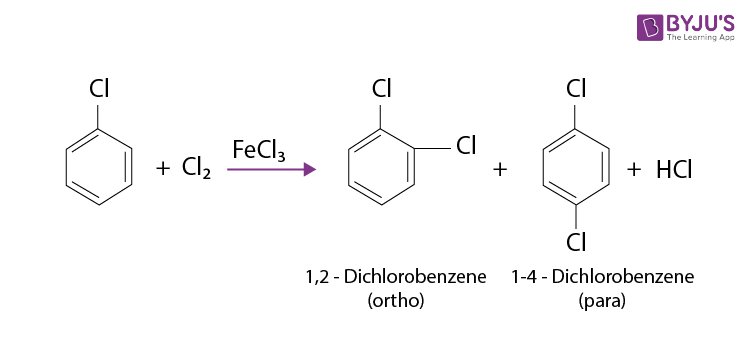
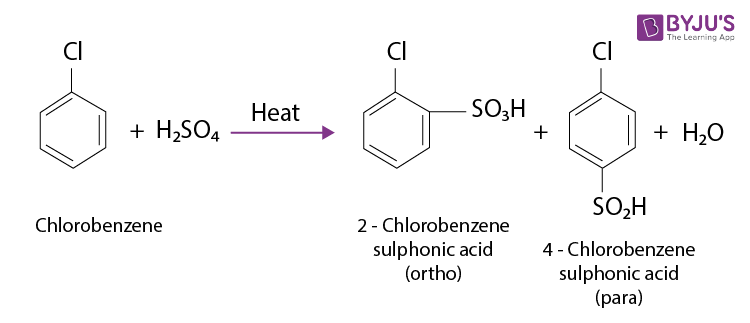
Resonance means that the halogen can donate some of its electron pairs back into the benzene ring. This actually increases the electron density at certain positions on the ring, specifically the ortho and para positions (think of them as positions 2 and 4 on a clock face if the halogen is at the 12 o'clock position).
It's like Hal is being a bit of a jerk by hogging the snacks, but then he also brings a cooler full of even tastier treats to share... but only with certain guests! So confusing!
However, the inductive effect is generally stronger than the resonance effect in haloarenes. So, overall, the halogen still deactivates the benzene ring, making it less reactive towards electrophilic substitution.
The Destabilizing Effect
Think of the reaction as a climb up a hill. The top of the hill is the transition state – the highest energy point in the reaction. To get the reaction to happen, you need to push it over that hill.
Now, haloarenes have a destabilizing effect on the transition state of electrophilic substitution. It's like adding extra weight to the climber's backpack, making it harder to reach the top of the hill. This means you need more energy to get the reaction going, which translates to a slower reaction rate.
The destabilization happens because the positive charge that develops in the transition state interacts unfavorably with the electron-withdrawing halogen.
So, How Slow Are We Talking?
Okay, so we know haloarenes react slower. But just how slow are we talking? Well, imagine trying to run a marathon in quicksand! It's not going to be a speedy race, is it?
Compared to benzene itself, haloarenes can react significantly slower in electrophilic substitution reactions. This means you might need higher temperatures, stronger catalysts, or longer reaction times to get the reaction to proceed at a reasonable rate.
It's like you have to bribe Hal with extra-delicious snacks and play his favorite music really loud just to get him to even tap his foot to the beat!
Orientation Matters!
But here's a twist! While the halogen deactivates the ring overall, its resonance effect also dictates *where* the incoming electrophile will likely attach.
Because the halogen increases electron density at the ortho and para positions, the electrophile is more likely to substitute at those locations. We say that halogens are ortho, para-directing groups.
It's like Hal is slowing down the party, but he's also subtly guiding certain guests to specific spots on the dance floor!
"So, even though haloarenes are sluggish, they still have their own unique way of controlling the party!"
Examples in the Real World
Why should you care about all this? Well, haloarenes are used in all sorts of things! From pharmaceuticals to pesticides, they're essential building blocks in many important molecules.
Understanding how their reactivity is affected by the halogen is crucial for chemists to design and synthesize these compounds effectively. It's like knowing how to handle Hal at a party to make sure everyone still has a good time, even if things are moving a bit slower!
For example, certain herbicides contain chlorinated aromatic rings. The presence of chlorine affects their interaction with plants and the environment. Understanding the electrophilic substitution behavior helps scientists predict their fate in the ecosystem.
In Conclusion: Halogens – Slow but Steady
So, there you have it! Haloarenes react slowly in electrophilic substitution reactions because of a combination of the inductive effect (electron-hogging), resonance (a bit of electron-donating, but not enough to win!), and the destabilization of the transition state.
While they might be the party dampeners, they also play a vital role in directing the reaction to specific positions on the benzene ring.
Just remember, even the slowest dancers can still bring something unique to the party! Embrace the "Hal" in your life, and understand that even slow and steady can win the race (or, in this case, the chemical reaction)!
Now go forth and impress your friends with your newfound knowledge of haloarene reactivity! You're officially a haloarene party expert!