Okay, so picture this: you're at a party, right? And everyone's just milling about, kind of doing their own thing. That, my friend, is kind of like a gas. But what happens when you crank up the music, serve some super strong coffee, and everyone starts *really* getting into it? Things get… energetic. That's plasma!
Seriously though, we're talking about states of matter here. You know, like solid, liquid, gas... and the often-forgotten rockstar, plasma.
Gas: The Chill Dude
A gas, simply put, is a bunch of atoms or molecules just bouncing around. They've got enough energy to not be stuck together like a solid or a liquid, but they’re still pretty chill. Think of air! You can't see it (usually!), but it's all around you, doing its gas thing.
They're not really interacting much; just bouncing, colliding, and generally minding their own business. It's like a polite cocktail party where everyone sticks to their own small groups. Bor-ing! (Sorry, gas. We still love you.)
Want to make a gas? Just heat up a liquid enough. Water becomes steam. Voila! Gas.
Plasma: The Party Animal
Now, plasma... oh, plasma is a whole different story. Plasma is basically a gas that's been heated up so much that the electrons get ripped away from the atoms. Boom! Suddenly, you've got a soup of positively charged ions and negatively charged electrons, all zipping around like crazy!
Imagine that same cocktail party, but someone just set off a confetti bomb and everyone's now doing the Macarena. That's plasma. Chaos! (In a good way, usually.)
Think about it: regular gases are electrically neutral. But plasma? Plasma is *charged*. This is a *big* deal because charged particles interact with magnetic fields. Like, *really* interact. Ever seen those cool plasma globes where the lightning bolts follow your finger? That's the magnetic field at work!
Want to make plasma? Crank up the heat even MORE! We're talking *extremely* hot. We're talking temperatures that would melt pretty much anything you can think of. Think lightning, the sun, and those super cool welding torches you see on TV.
So, What’s the Actual Difference?
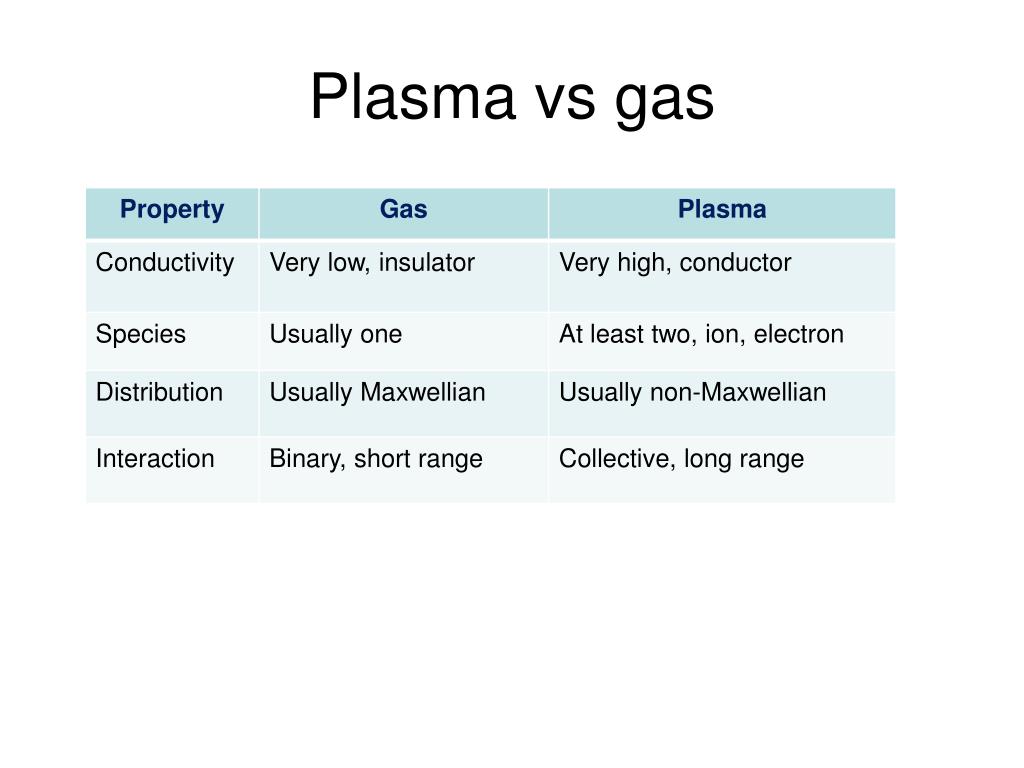
Alright, let's break it down: the key difference between plasma and a gas is ionization. In a gas, the atoms are mostly neutral. But in plasma, the atoms have been ionized, meaning they've lost electrons and become charged ions.
Here's a handy-dandy (and probably slightly oversimplified) table:
- Gas: Neutral atoms/molecules, relatively low energy, not strongly influenced by magnetic fields.
- Plasma: Ionized gas, extremely high energy, strongly influenced by magnetic fields.
See? Easy peasy!
Why Should I Care?
Good question! Why *should* you care about the difference between gas and plasma? Well, for starters, plasma is actually the most common state of matter in the universe. I'm serious! Stars? Plasma. The solar wind? Plasma. Interstellar space? You guessed it: plasma!
But even here on Earth, plasma is used in all sorts of cool stuff. Think about neon signs (yep, that's plasma!), plasma TVs (okay, maybe not so much anymore, but they were cool!), and even some types of industrial welding. So, it's not just some abstract, sci-fi concept. It's all around us!
And who knows, maybe *you'll* be the one to discover the next groundbreaking application of plasma! (Okay, probably not. But hey, a guy can dream, right?)
So, there you have it. Gas: chill. Plasma: party. Got it? Great! Now, who's buying the next round of coffee?