Hey there, chemistry enthusiast! Ever stumbled upon a funky-looking molecule and thought, "What *even* is that thing?" Today, we're diving into the wonderful world of hemiacetals, acetals, and… well, other stuff. Consider this your cheat sheet to decoding those molecular mysteries!
Forget boring lectures. Think of this as a molecular cocktail party. We're just here to mingle and learn a few fun facts.
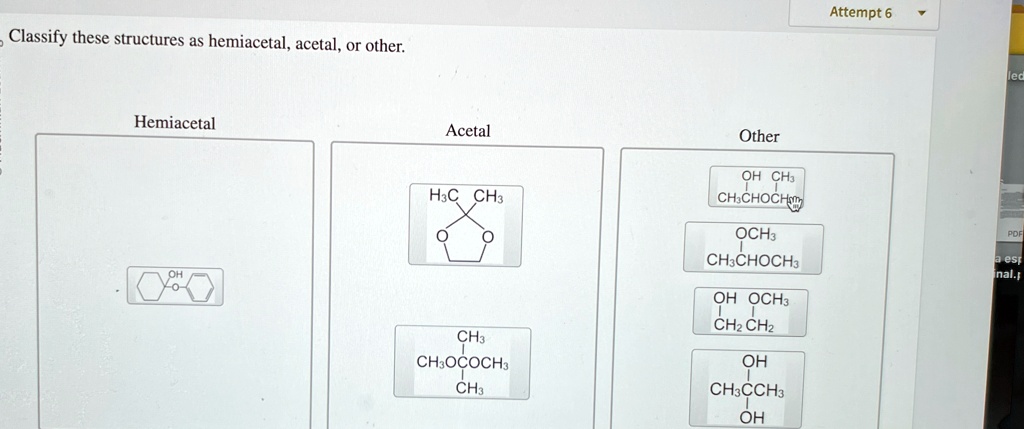
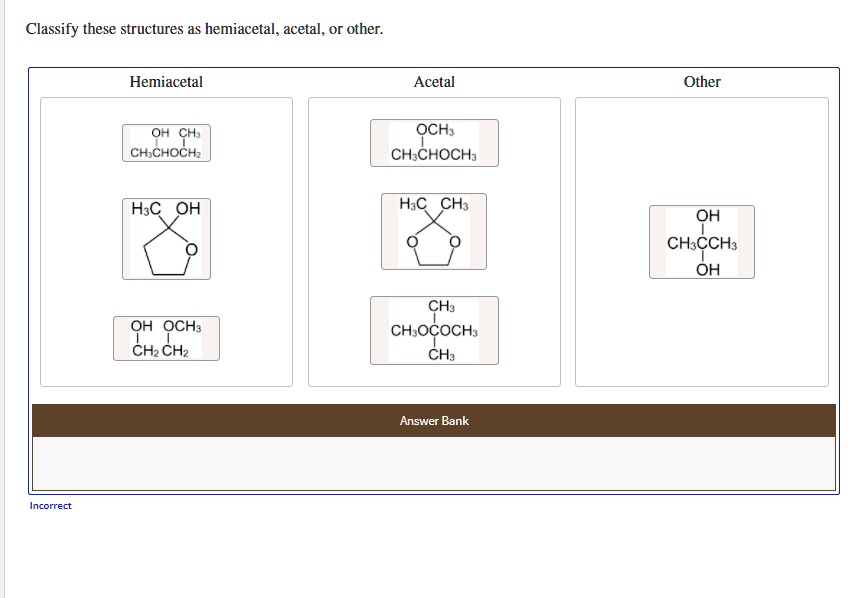
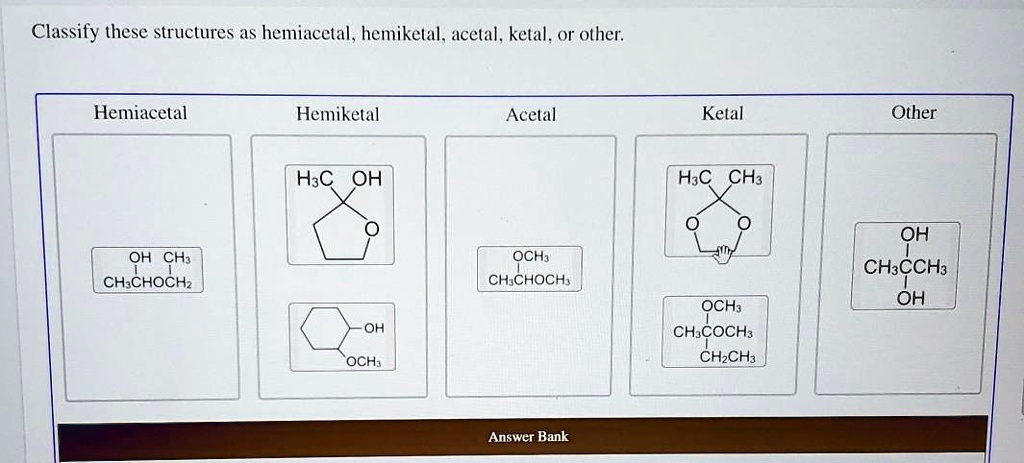
What's the Deal with Hemiacetals?
Imagine a half-formed acetal. That's pretty much what a hemiacetal is. It’s like the awkward teenager of organic chemistry – not quite one thing, not quite another.
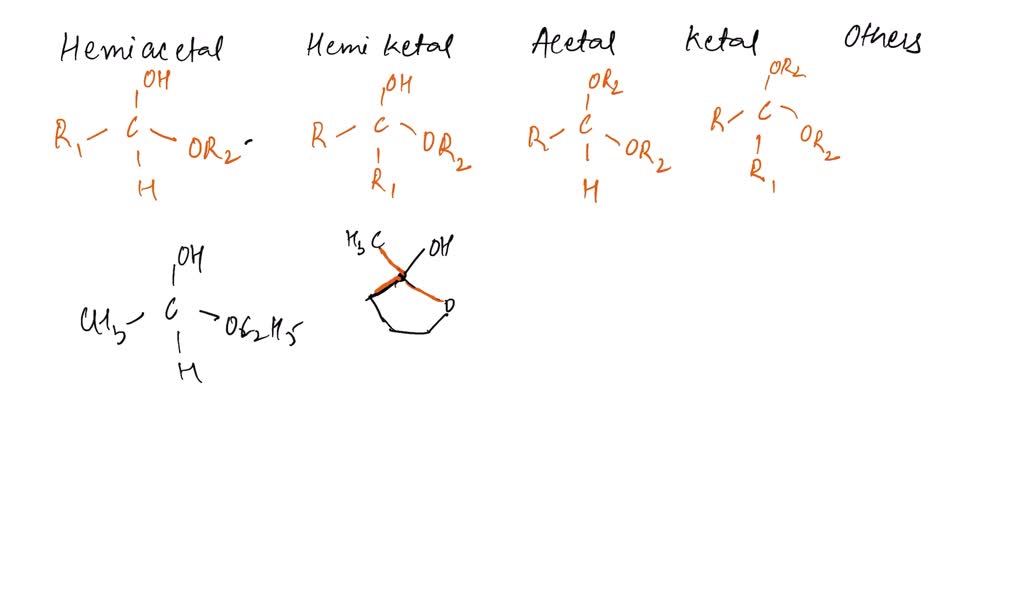
The key ingredients for a hemiacetal are an alcohol (ROH) and an aldehyde or ketone (RCHO or RCOR). They get together in a single carbon atom, which also ends up sporting an ether group (OR) and a hydroxyl group (OH). Got it? Great!
Here's a quirky fact: Hemiacetals are often fleeting. They're unstable intermediates, meaning they don't stick around for long. They're more like a quick hello than a lasting relationship. Think of them as the mayflies of the molecular world, beautiful but brief!
So, if you see a carbon with both an -OH and an -OR group attached, and it used to be part of a carbonyl, you've likely spotted a hemiacetal. Congrats, you're practically a molecular detective!
And What About Acetals?
Acetals are the fully formed, more stable cousins of hemiacetals. They've fully committed to the bit. They're the life of the party, always reliable, and generally well-behaved (chemically speaking).
To get an acetal, you need a hemiacetal to react with another alcohol molecule. Poof! Water gets kicked out, and you end up with a carbon bonded to two ether groups (-OR and -OR). Double the ether, double the fun!
Acetals are like molecular protective gear. They're often used to shield aldehydes or ketones during reactions. Because acetals are relatively unreactive, they can protect your carbonyl while you mess around with other parts of the molecule. Clever, right?
Funny detail: Acetals can be *reverted* back to aldehydes or ketones by adding water under acidic conditions. It's like undoing all your hard work...but in a controlled, scientifically useful way!
"Other": When Things Get Weird
Now, let's talk about the "other" category. This is where things get interesting. This is where the rule-breakers and the oddballs hang out.
Maybe you’ll find a molecule that *looks* similar to a hemiacetal or acetal, but it's missing a key component. Perhaps it’s a completely different functional group altogether. The possibilities are endless!
For instance, maybe the molecule has an -OH group attached to a carbon, but that carbon wasn't originally part of a carbonyl. Or maybe it's a ketal – the ketone version of an acetal. Same concept, different starting material. Don't let it throw you!
Remember the bigger picture: Look for the key functional groups. Identify the key atoms. Trace the molecule's history (hypothetically, of course). Did that carbon used to be a carbonyl? Is there an -OH group? Are there ether linkages?
Decoding Molecular Structures: Tips and Tricks
Feeling overwhelmed? Don't be! Here are a few tips to keep in your back pocket:
- Focus on the Carbon: The central carbon atom is the key to identifying these structures. What's attached to it?
- Look for -OH and -OR: These are the telltale signs of hemiacetals and acetals.
- Think about Stability: Hemiacetals are generally unstable, while acetals are more stable.
- Draw it Out: Sometimes, the best way to understand a molecule is to sketch it out yourself.
Consider this analogy: imagine you're trying to identify different types of sandwiches. A hemiacetal is like a half-finished sandwich, an acetal is a complete sandwich, and "other" is everything else – a salad, a pizza, a bowl of soup. You get the idea!
Identifying hemiacetals, acetals, and “other” structures isn't just about memorizing definitions. It's about developing a molecular intuition. It’s about training your eye to spot patterns and recognize functional groups. It's about becoming a true chemistry connoisseur.
So, next time you encounter a mysterious molecule, don't panic. Take a deep breath, apply your newfound knowledge, and remember: chemistry is just a giant puzzle waiting to be solved. And who doesn’t love a good puzzle?
Now go forth and classify! You've got this!