Hey there, science buddies! Ever wondered about those rockstars of the periodic table? You know, the elements that are just *itching* to react with everything and look good doing it? Well, buckle up, because we're diving into the world of an element that's highly conductive, ridiculously reactive, surprisingly soft, and oh-so-lustrous!
Think of it as the element that's always ready for a party and dressed to impress. It's like the social butterfly of the atomic world, but instead of gossip, it's swapping electrons! (Okay, maybe there's a *little* atomic gossip, we don't know for sure!).
Meet the Alkali Metals (Probably!)
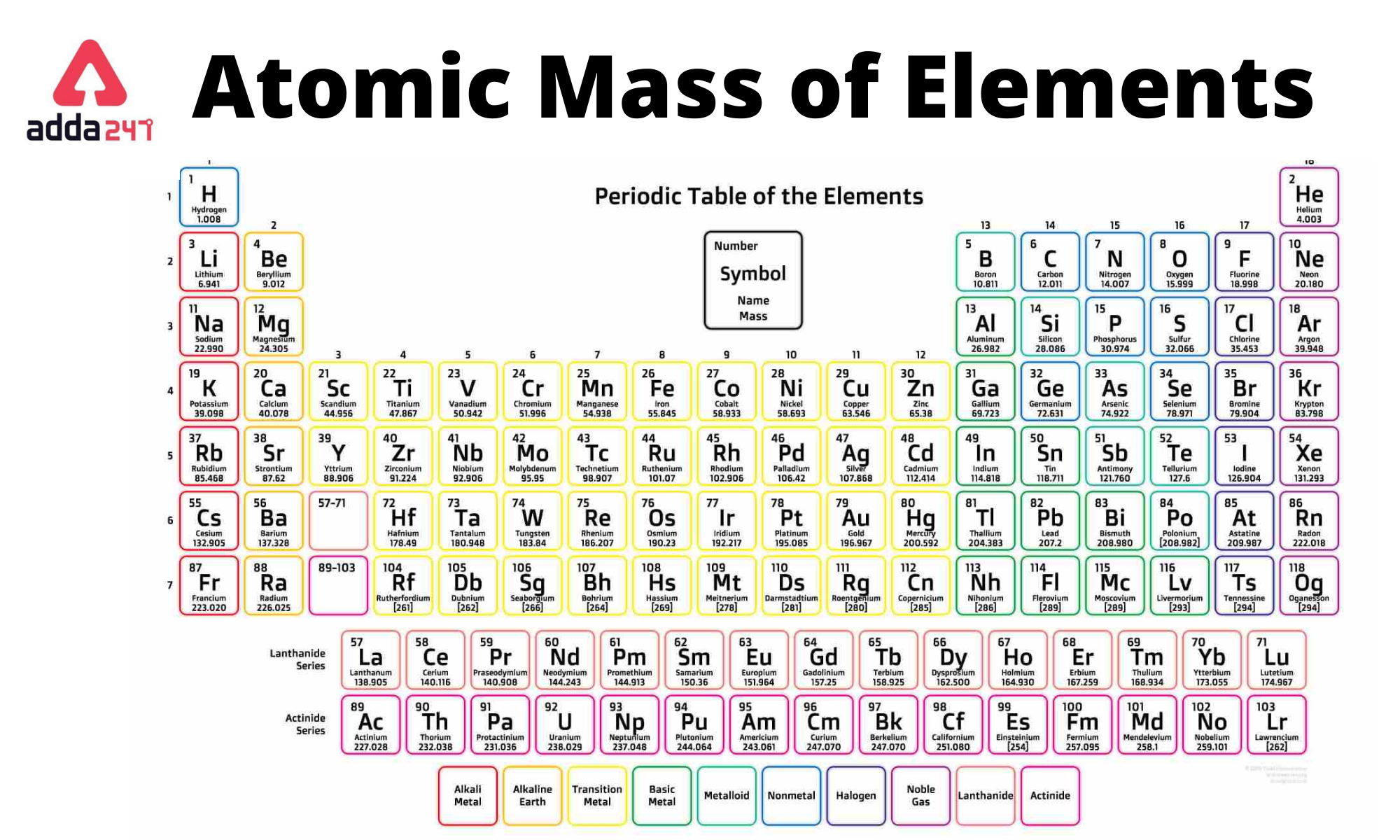
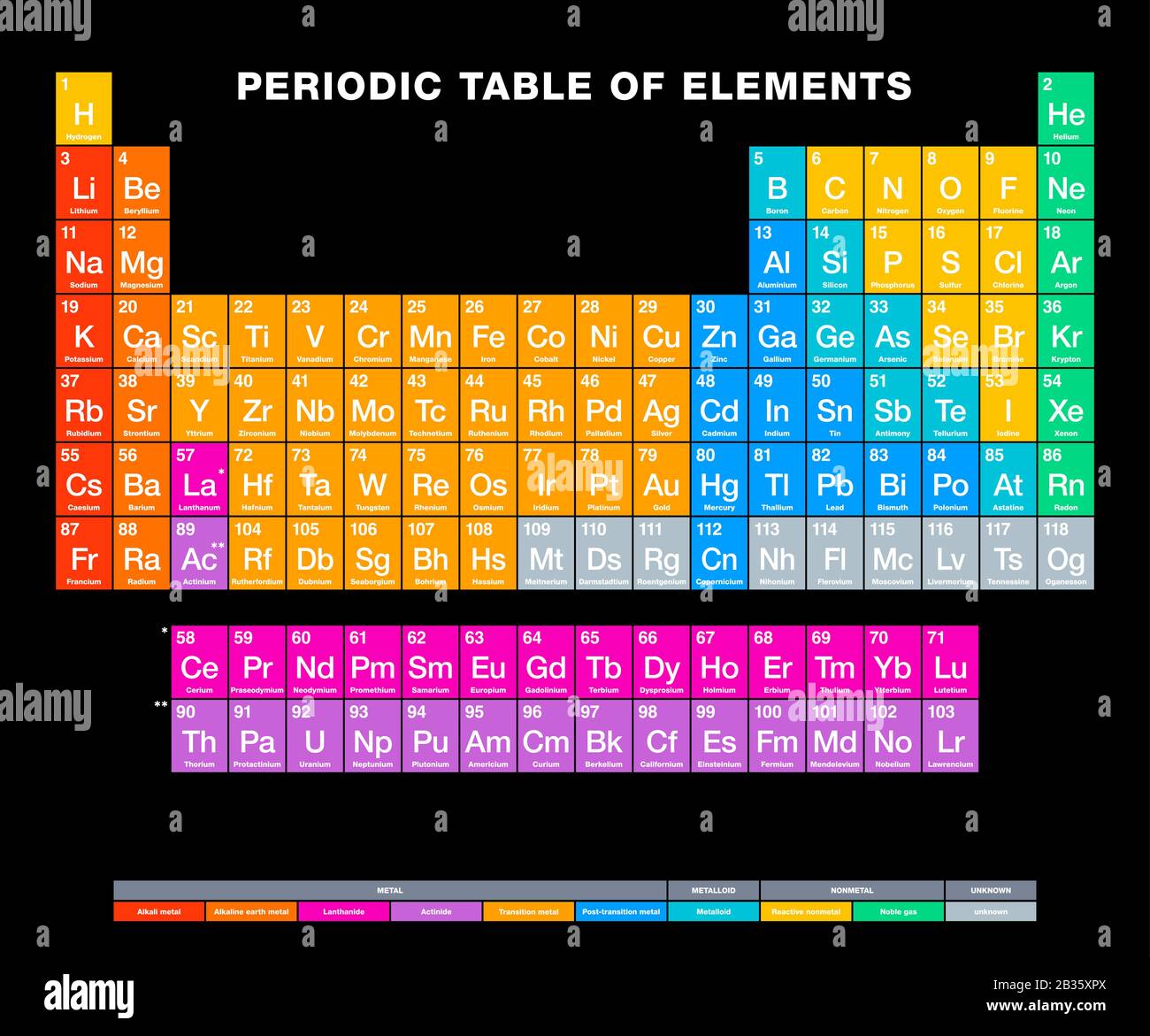
Now, I'm not going to name names (mostly because there are a few contenders!), but chances are, we're talking about one of the alkali metals. These guys are located in the first group of the periodic table, and they're notorious for their energetic personalities. Seriously, keep them away from water unless you want a *boom* kind of situation. Think of it as a really, *really* enthusiastic bath time.
Why so reactive? It all boils down to their electron configuration. They have just one lonely electron in their outermost shell, and they're just dying to get rid of it to achieve a more stable, noble gas-like state. It's like having that one awkward tie you can't wait to donate to Goodwill. They just *need* to get rid of it!
So, when they encounter another element that's electron-hungry (like chlorine, for example), they're like, "Take it! Take it all! Just give me stability!" And then...bam!...a chemical reaction occurs, often releasing a whole lot of energy in the process.
Conductivity: The Power of Flow
Besides being reactive, our mystery element is also highly conductive. This means it's great at letting electrons flow through it. Imagine a superhighway for tiny, negatively charged particles. That's basically what's happening inside this element.
This conductivity stems from the fact that those outer electrons aren't held very tightly. They're kind of like free agents, wandering around and readily carrying an electrical current. This makes them useful in all sorts of applications, from electrical wiring (though other elements are generally preferred for safety!) to specialized batteries.
Softness and Shine: Beauty and the Bond
Now, for the surprising part: our reactive, conductive pal is also soft! You can often cut them with a knife! (Please don't try this at home unless you're a trained chemist with appropriate safety precautions. Seriously, we don't want any alkali metal-related kitchen accidents!).
This softness comes from the relatively weak metallic bonds holding the atoms together. Remember that one lonely electron? It's not doing a whole lot to contribute to a strong, cohesive structure. It's like trying to build a sturdy wall with only a few pebbles. Not gonna happen.
And finally, let's talk about the lustre! Freshly cut, these elements are beautifully shiny and reflective. This is because the free-flowing electrons can easily absorb and re-emit light. It's like a tiny, metallic disco ball, reflecting light in all directions.
However, that shine doesn't last long! Because they're so reactive, they quickly tarnish when exposed to air, forming an oxide layer on the surface. It's like putting your disco ball in the rain – not a good look.
So, What's the Point?
Okay, so we've learned that this element is reactive, conductive, soft, and shiny. But what's the big deal? Well, it illustrates a fundamental principle of chemistry: the properties of an element are determined by its atomic structure, particularly its electron configuration. Everything is connected!
And more importantly, it shows us that even seemingly contradictory properties can coexist. Something can be both beautiful and dangerous, reactive and useful. It's a reminder that the world is full of fascinating complexities, just waiting to be explored.
So, the next time you see something that seems simple on the surface, remember the alkali metals and their quirky personalities. There's always more than meets the eye. And who knows? Maybe you'll discover your own inner reactivity and shine, ready to connect with the world and make a little (or a big!) impact. Go forth and react!



/periodic-table-of-the-elements-2017--illustration-769723031-5ac10eb6a9d4f9003769784d.jpg)