Ever heard of the alkali metals? They're a rowdy bunch on the periodic table! Think of them as the rockstars of chemistry, always ready to party (or, you know, react).
What Makes Them Special?
These metals are all about sharing... well, *losing* an electron. That single electron makes them incredibly reactive. It's like they're desperate to get rid of it!
Physical Properties: Shiny, Soft, and Speedy
First off, alkali metals are usually shiny. Freshly cut, they gleam! But don't expect that shine to last long. They tarnish quickly in air.
They're also surprisingly soft. You can cut them with a knife! Try that with iron… good luck!
Here's another cool thing: they have low densities. Lithium, sodium, and potassium are less dense than water! Imagine a metal that floats!
Their melting and boiling points aren't crazy high either. This is because the metallic bonds holding them together aren't super strong. It's all about that one lonely electron!
Chemical Properties: Ready to React!
This is where the fun really begins. Alkali metals are *extremely* reactive.
Remember that single electron they're trying to ditch? That's what drives their reactions. They're always looking for something to give it to.
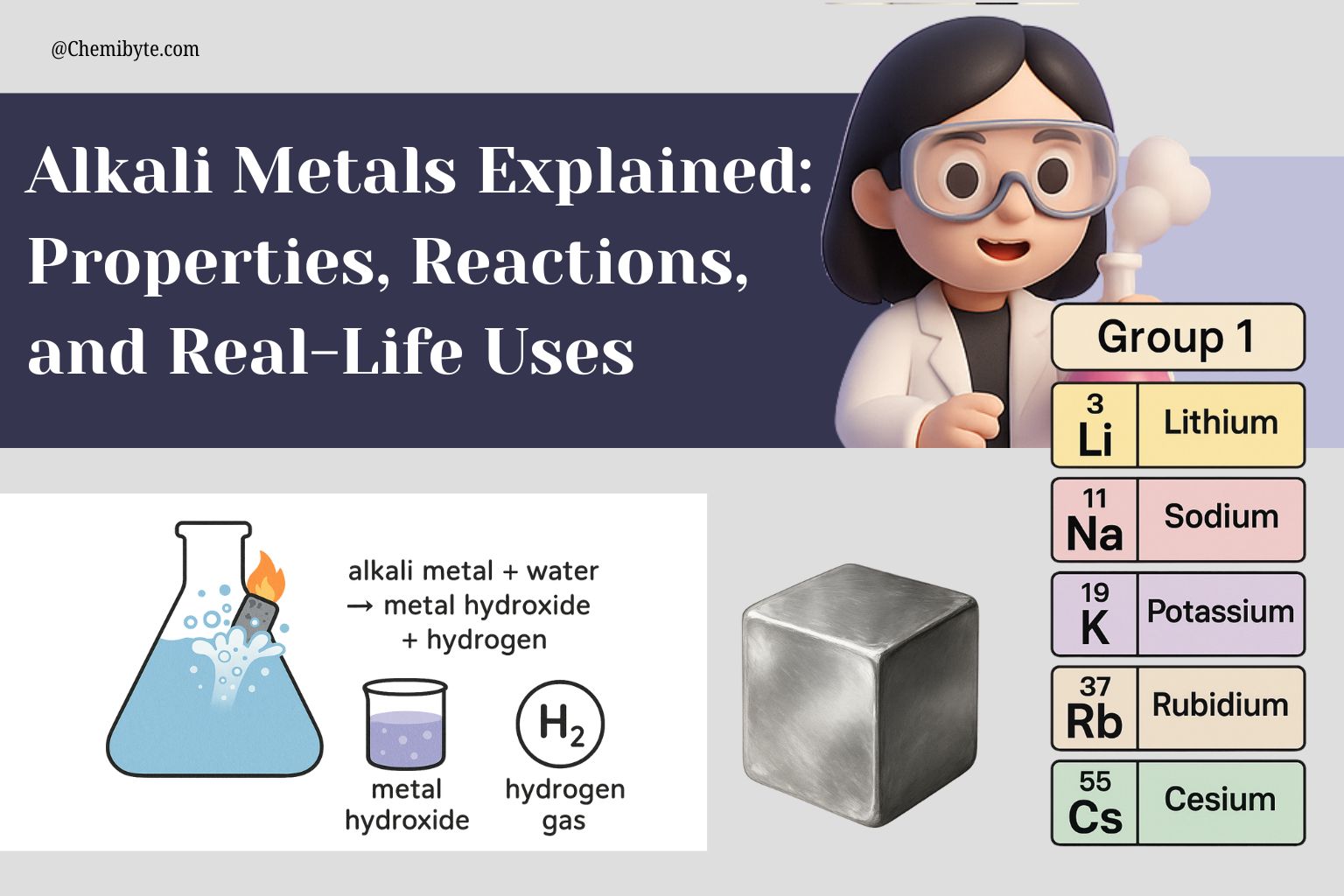
They react vigorously with water. Sometimes even explosively! Don't try this at home, folks! These reactions release hydrogen gas and heat. Big booms can occur!
They also love to react with halogens (like chlorine and fluorine). This forms salts, like sodium chloride (table salt). A classic example of opposites attracting!
The reactivity increases as you go down the group. Cesium is more reactive than sodium, for example. That extra electron is even further from the nucleus, making it even easier to lose.
The Alkali Metal Lineup
Let's meet the band members, shall we? Each one has its own personality (and uses!).
Lithium (Li): The Lightest One
Lithium is the lightest of the alkali metals. It's used in batteries! Think about your phone or laptop – lithium is probably powering it.
It's also used to treat bipolar disorder. A little bit of lithium can go a long way!
Sodium (Na): The Table Salt Star
Sodium is probably the most familiar alkali metal. It's a key component of table salt (sodium chloride). We sprinkle it on our food every day!
It's also important for nerve function and muscle contraction. Sodium helps our bodies work properly.
Potassium (K): The Banana Booster
Potassium is another essential element for life. You get it from bananas! It's crucial for maintaining fluid balance and nerve function.
It's also used in fertilizers. Potassium helps plants grow strong and healthy.
Rubidium (Rb): The Atomic Clock Contender
Rubidium is a bit more exotic. It's used in atomic clocks. These clocks are incredibly accurate!
It also has some niche applications in research and medicine. It's a bit of a specialist.
Cesium (Cs): The Reactive Ringmaster
Cesium is one of the most reactive metals on Earth. It reacts violently with water. It is used in some high-precision atomic clocks as well.
It also has some uses in photoelectric cells. Cesium can release electrons when exposed to light.
Francium (Fr): The Rare and Radioactive Rebel
Francium is super rare and radioactive. It doesn't stick around for long! It is very difficult to study due to its fleeting existence.
Scientists don't know a whole lot about it. It's a true mystery of the periodic table!
Why Are They So Entertaining?
The real entertainment comes from their reactivity. The way they explode (sometimes literally!) in water is captivating. It's a visual demonstration of chemistry in action.
Think of it like a controlled (and safe!) science experiment. You get to see matter transforming before your eyes. Plus, there’s usually a bang involved!
It's also fascinating to learn about their uses. From powering our phones to keeping our bodies healthy. Alkali metals play a surprisingly big role in our lives.
Safety First!
It's important to remember that alkali metals are dangerous. They should only be handled by trained professionals in a lab setting.
Don't try to recreate any of these experiments at home. Leave the fireworks to the experts!
Instead, explore the world of alkali metals through books, videos, and online resources. There's a wealth of information available to satisfy your curiosity.
The Takeaway
Alkali metals are a fascinating group of elements. They're reactive, shiny, and surprisingly useful. Their properties make them both entertaining and important.
So, the next time you see sodium chloride or hear about lithium batteries, remember the rowdy rockstars of the periodic table. They're always ready to put on a show!
Maybe you'll even be inspired to learn more about chemistry! The world of elements is full of surprises!