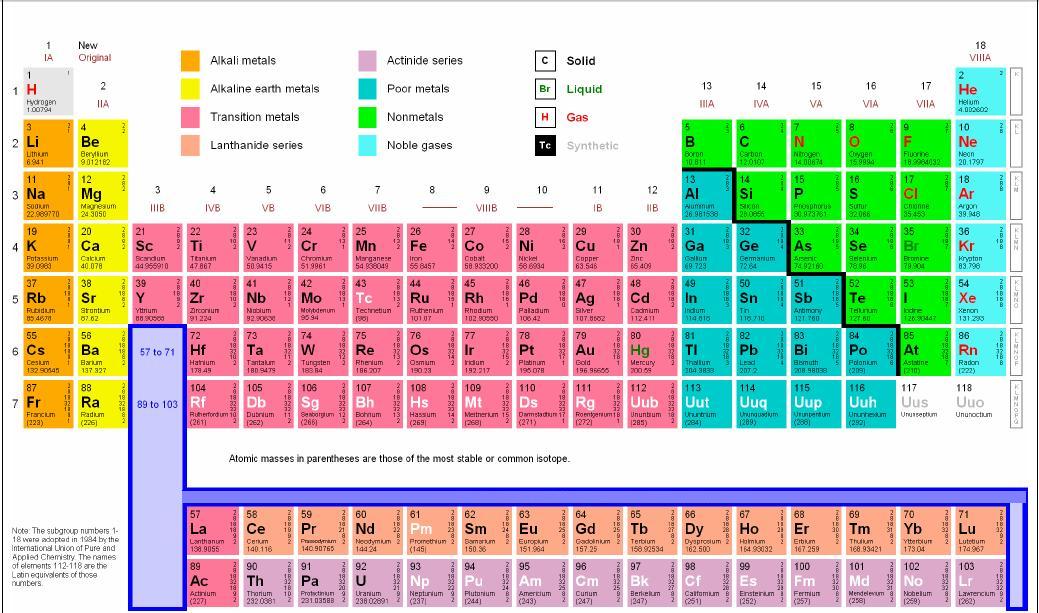
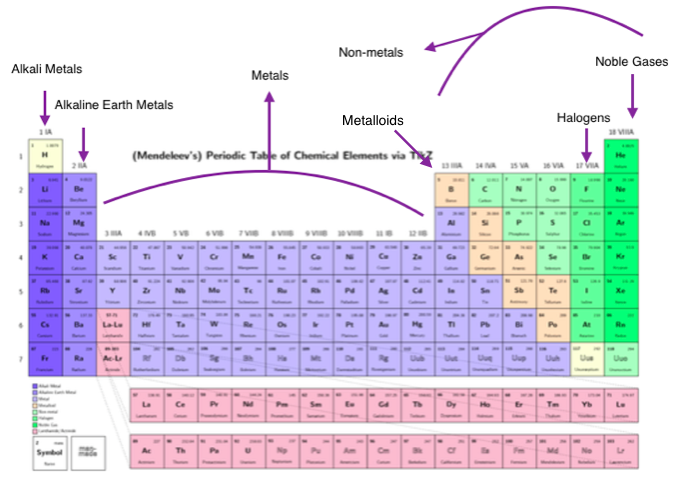
Hey! Grab a coffee, let's talk elements. Not just any elements, though. We're diving into the coolest groups on the periodic table – the Alkali Metals, Alkaline Earth Metals, Halogens, and Noble Gases. Buckle up, it's gonna be... well, elemental!
Alkali Metals: The Wild Ones
First up, the Alkali Metals! Think of them as the rockstars of the periodic table. Group 1, baby! (Minus Hydrogen, that little oddball). They are super reactive. Like, "throw them in water and stand back" reactive. Why? Because they only have one lonely electron in their outer shell, just itching to get rid of it.
Lithium, Sodium, Potassium... you've probably heard of them, right? Sodium's in table salt, and Potassium is that banana nutrient everyone raves about. But Cesium and Francium? Now those are the really crazy ones. Don't even THINK about messing with Francium unless you're a trained professional... or you *want* to go boom.
Seriously, these guys are soft (you can cut them with a knife!), shiny (briefly, before they tarnish), and ready to party (by which I mean react explosively). Want to impress your friends? Tell them Alkali Metals reactivity increases as you go down the group. Mind. Blown.
Alkaline Earth Metals: Less Wild, Still Cool
Next, we have the Alkaline Earth Metals. Group 2! These guys are like the Alkali Metals' slightly more responsible cousins. Still reactive, but not quite as "hold my beer" as their Group 1 buddies. They have two electrons in their outer shell, so they're still eager to bond, just not as desperately.
Magnesium is in your Epsom salts, Calcium builds strong bones, and Barium... well, Barium is used in X-rays, because it's good at blocking them. Ever heard of Beryllium? It's super strong but also toxic – kinda like a superhero with a dark secret. Interesting, right?
Again, reactivity increases down the group. But they’re also generally harder and denser than Alkali Metals. So, tougher cousins, got it?
Halogens: The Electron Thieves
Now, let’s get a little evil. Meet the Halogens! Group 17. These guys are electron thieves. They are desperate to get one more electron to complete their outer shell. Think of them as the popular kids who are always asking to borrow your notes... and never giving them back.
Fluorine, Chlorine, Bromine, Iodine... sound familiar? Chlorine's used to disinfect pools (that's why they smell like chlorine!), and Iodine is in that antiseptic you put on cuts. Fluorine keeps your teeth strong! (Thanks, Halogens!). Astatine, though, is another rare and radioactive one. Stay away unless you know what you're doing!
They're highly reactive (seeing a pattern here?). They like to form salts (hence the name "halogen" which means "salt-former"). And their reactivity *decreases* as you go down the group, so Fluorine is the biggest bully on the block. Good to know!
Noble Gases: The Aloof Celebrities
Last but not least, the Noble Gases! Group 18. The celebrities of the periodic table. They have a full outer shell of electrons, meaning they're perfectly happy and don't need to bond with anyone. They're the "I don't need you, I'm complete on my own" type. Aloof? Maybe. Stable? Definitely.
Helium (balloons!), Neon (signs!), Argon (light bulbs!)... these guys are everywhere. They’re inert, meaning they don't react easily. Makes them great for creating safe environments or those bright, colorful signs that scream “OPEN!” at 2 AM.
While they used to be considered *completely* unreactive, scientists have managed to coax a few of them into forming compounds under extreme conditions. Turns out even celebrities have their moments of weakness, huh? But mostly, they like to keep to themselves. They’re noble, after all.
So there you have it! A whirlwind tour of some of the coolest groups in the periodic table. Now you can impress all your friends with your newfound knowledge of Alkali Metals, Alkaline Earth Metals, Halogens, and Noble Gases! Go forth and element-splain! (I just made that word up. You're welcome.)